Table 2. Substrate scope for benzylic Csp3–H arylation of N-benzylamines a .
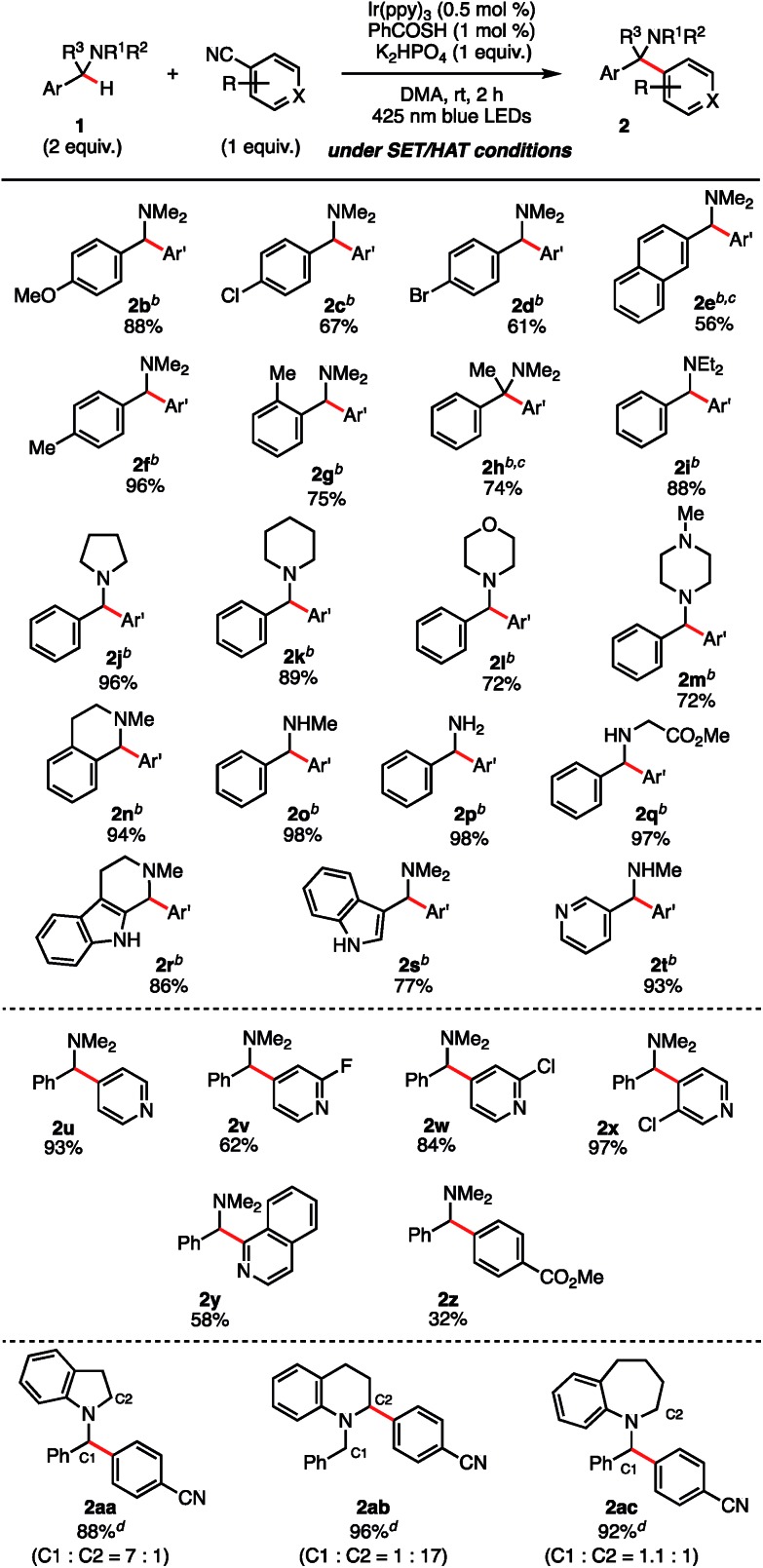
|
aAll reactions were conducted on 1 mmol scale.
bTerephthalonitrile was used as an arylating reagent (Ar′ = 4-NCC6H4).
cThe reaction was carried out for 6 h.
dYield and regioisomeric ratio were determined by 1H NMR analysis using 1,1,2,2-tetrachloroethane as an internal standard.
