Abstract
The solar radiation range has harmful and beneficial effects. Sunscreens, which selectively block specific spectral regions, may potentially interfere with skin homeostasis. For instance, the ultraviolet (UV) B waveband produces erythema and DNA damage; simultaneously, it induces pre-vitamin D3 synthesis. UVA1 and visible light can both induce pigmentation in skin phototypes IV–VI, and act in synergy to induce erythema and persistent pigment darkening. In contrast, UVA may contribute to blood pressure control and cardioprotection by inducing release of nitric oxide from intracutaneous photolabile nitric oxide derivatives. Finally, infrared A radiation alters the collagen equilibrium of the dermal extracellular matrix but is involved in the regulation of body temperature and in nitric oxide release, with a potential beneficial impact on blood pressure regulation. Ideally, photoprotection should thus be performed with a neutral density filter, mitigating all radiation ranges homogeneously, to maintain solar spectrum homeostasis. Natural compounds such as mycosporine-like amino acids are promising natural UV radiation-filtering compounds for an improved homeostasis with our environment. Lastly, we should not forget individual characteristics and behavior, as homeostasis differs according to individual phototypes and skin exposure behaviors.
Key Points
| Spectral homeostasis is a desirable goal because all solar radiation (ultraviolet, infrared, and visible light) can both affect the skin and have beneficial effects contributing to human health. |
| The ideal photoprotector to maintain solar spectrum homeostasis should be a neutral density filter mitigating all radiation ranges homogeneously. |
Introduction
The concept of ‘spectral and skin homeostasis’ applied to sunscreens is a developing notion, which will impact on topical formulations in the near future. It refers to the fact that the continuous use of sunscreens that filter particular solar wavelengths may be altering skin homeostasis on a daily basis.
Spectral homeostasis, in particular, would mean leaving the natural solar spectrum unchanged, even though attenuated by sunscreen, clothing, or shading structures. Spectral homeostasis is a desirable goal for two reasons: (1) not only ultraviolet (UV) radiation (UVR), but also infrared radiation (IR) and visible light (VL), i.e., the total solar spectrum, can affect the skin; and (2) the biological effects of solar radiation also include beneficial effects, contributing to human health. This article focuses on the beneficial aspects of solar electromagnetic radiation (EMR) on the skin and the recent developments of research on this topic.
Solar Exposure and Skin Homeostasis
The solar radiation reaching the top of the earth’s atmosphere is composed of only 5–7% of UVR, which covers the wavelength range 100–400 nm and is divided into three bands: UVC (100–280 nm), UVB (280–315 nm), and UVA (315–400 nm) [1, 2]. Through the atmosphere, all UVC and approximately 90% of UVB radiation is absorbed, so that the UVR reaching the earth’s surface is mainly composed of UVA, with a small UVB fraction. Visible radiation, which covers the wavelength range 400–780 nm, accounts for 45% of solar radiation, while IR, composed of wavelengths longer than 780 nm, represents the remaining 48–50%.
UV, VL, and IR radiation have different energy values and degrees of penetration into the skin [3]. Cutaneous effects are produced by the whole spectrum of EMR, in the UV, VL, and IR ranges, and may thus produce negative effects at all levels of the skin from the epidermis to the deep dermis [4].
Ultraviolet (UV) Exposure and Skin Homeostasis
Excessive solar exposure of the skin can lead to different types of damage, including sunburn (erythema), photoaging, and skin cancer. Erythema is an important factor in the pathogenesis of melanoma and keratinocyte skin cancers [5]. As such, UVR protection by various measures, including sunscreen, is largely recommended by international guidelines [6] and the World Health Organization (WHO) has defined a UV index (UVI) to encourage photoprotection in skin cancer prevention campaigns [1].
UVB Radiation and Pre-Vitamin D Biosynthesis
The influence of UVB has been the subject of much debate. The strict UVB protection recommended by several guidelines is now questioned, since it could be associated with vitamin D deficiency [7–10]. Dietary supplementation with low-dose vitamin D (< 400 IU) during the whole year can at least partly counteract the lack of solar exposure by maintaining adequate levels (25-OH vitamin D of 50 nmol/L) or reduction of winter declines [11].
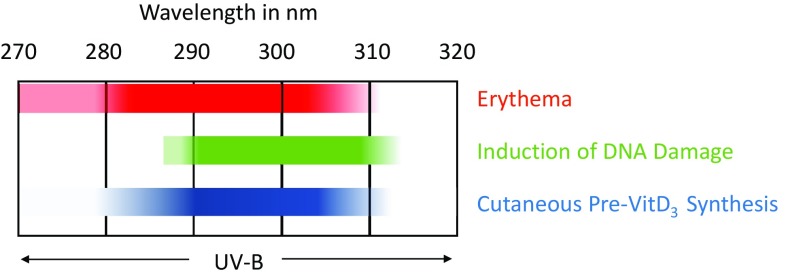
The fact that the action spectra (wavelength dependence) of both harmful (erythema, DNA damage induction) and beneficial phenomena (cutaneous pre-vitamin D3 synthesis) overlap (Fig. 1) questions the logic of a long-lasting evolutionary process of gene interaction with the environment. However, this apparent dilemma can be understood: (1) pre-vitamin D3 production does not require long periods of UVB irradiation; and (2) UVB radiation overexposure induces sunburn, a painful warning that prompts subsensitive individuals to reduce or avoid sun exposure.
Fig. 1.
Overlap between beneficial (cutaneous pre-vitamin D3 synthesis [41]) and harmful action spectrum (erythema action spectrum [42] and induction of DNA damage [43])
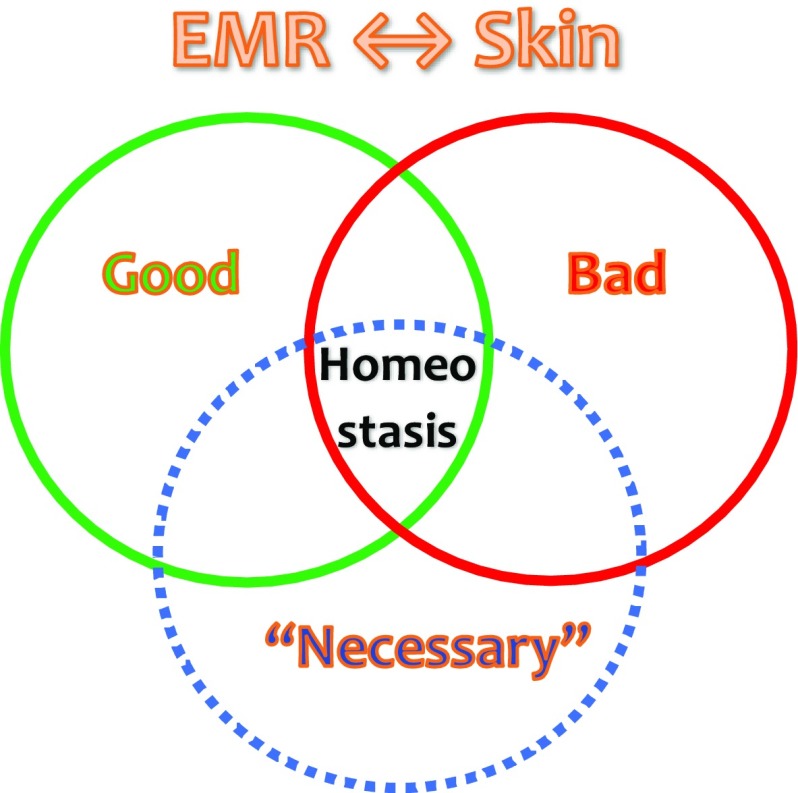
Since UVR is both the best natural source of vitamin D and a major cause of skin cancer, there is thus a fine line in balancing these beneficial and harmful effects. As pictured in Fig. 2, homeostasis stands at the crossroads between the good, the bad, and the necessary effects of EMR. This has led to new approaches to develop optimized sunscreens that enable vitamin D formation with reduced erythema risk [12].
Fig. 2.
Homeostasis of solar radiation. Between electromagnetic radiation (EMR) and the skin, homeostasis can be found at the intersection between good, bad, and ‘necessary’ effects of solar radiation
UVA Radiation and Blood Pressure
Independently of vitamin D production, UVR can also play a role in health via other mechanisms [13], such as improvement of mood through the release of endorphins [14] and alleviation of the symptoms of multiple sclerosis [15]. In particular, epidemiological data show than sun exposure is inversely correlated with the incidence of hypertension and cardiovascular disease mortality [16] and this hypotensive effect is not related to vitamin D, since oral vitamin D supplementation does not influence blood pressure patterns [17]. In Southern Europe, 30 min of solar exposure at midday on a sunny day can induce vasodilatation of the arterial vasculature. This effect is UVA-mediated and independent from skin temperature and warming by the sun [18]. Insights into the mechanism were brought by Oplander et al. in 2009 [19]. They showed that whole-body UVA exposure lowers systemic blood pressure by release of nitric oxide (NO) from intracutaneous photolabile NO derivatives, and that UVA skin irradiation can increase plasma nitrite (NO2) levels by 40%.
The role played by the epidermis is linked to its very rich content in cysteine-containing proteins. Their sulfhydryl groups readily undergo nitrosation to form endogenous nitrite and S-nitrosothiols (RSNOs). Nitrate (NO3), NO2, and RSNOs are present in the dermis and epidermis at concentrations one or two orders of magnitude higher than in plasma [20].
NO can act through several mechanisms. Palmer et al. [21] showed as early as 1987 that NO release accounts for the biological activity of endothelium-derived relaxing factor (EDRF). Besides, NO can also play a role on the peripheral vascular resistance via the differential expression of the hypoxia-inducible factor-α (HIF-α) isoforms in the skin, which are correlated with the degree of idiopathic hypertension in humans [22].
Many questions remain, such as the importance of UVA-mediated photolysis in blood pressure control, precisely how different wavelengths interact with NO-related species, and what is the subsequent fate of the reaction products? Is this UVA-mediated mechanism effective independent of age and gender in hypertensive individuals, through repeated stimulus [23]? However, since high blood pressure is the leading cause of premature death and disease worldwide, according to the WHO’s most recent survey [24], the hypotensive effect of UVA exposure [25] and the potential interference of UVA–skin interactions by sunscreens merits further investigation. Indeed, the UVA spectrum is responsible for > 80% of NO released [18].
Visible Light and Skin Homeostasis
Long thought to be ‘innocuous’, VL was recently shown to be able to induce persistent pigment darkening and DNA damage via reactive oxygen species and to exacerbate some photodermatoses. Although UVA1 (340–400 nm) can also induce pigmentation in skin phototypes IV–VI, VL-induced pigmentation is darker and more persistent [26]. Moreover, VL acts in synergy with UVA1 to induce erythema and pigmentation [27].
Infrared Radiation and Skin Homeostasis
IR is involved in several biological mechanisms. Although IRA (700–1400 nm) may decrease collagen synthesis in the dermal extracellular matrix by increasing the expression of the collagen-degrading enzyme, matrix metalloproteinase 1 (MMP-1), workers exposed to industrial sources of IR in amounts comparable to typical annual levels of IRA solar exposure do not experience significant long-term skin damage [28]. In addition, IRA is involved in the regulation of body temperature and in NO release, with a potential beneficial impact on blood pressure and possibly on cardioprotection [29]. There is therefore a need for quantitative analysis of the benefits, if any, of incorporating agents into sunscreens that reduce cutaneous IRA damage [30].
Sunscreen Spectrum Homeostasis
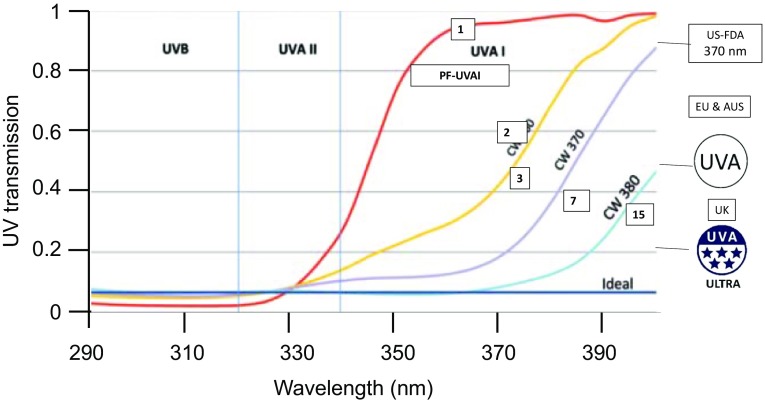
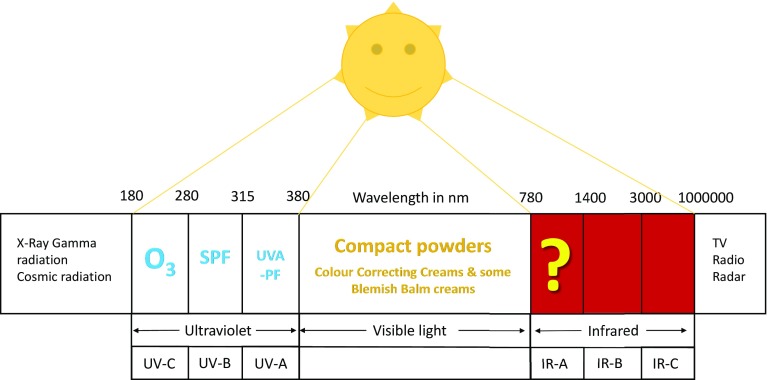
Sunscreens are used to provide ‘adequate’ protection against the damage induced by EMR. Current sunscreens, based on combinations of filters, are able to block almost all the UVR spectral range, nearly as efficiently as protection by clothing (Fig. 3) [31, 32]. Protection against VL can be provided by compact powders, which are added in blemish balm or color-correcting creams (Fig. 4). However, what about IR? No standardized tests provide a basis for the claims made for some products. Solar protection should not impede the beneficial functional skin responses, which result from EMR and skin interactions. The advent of the concept of skin homeostasis calls, therefore, for the development of sunscreens ensuring uniform spectral absorbance across the UV and global solar spectrum (Fig. 4). The recognition that the addition of VL and IRA to the regular solar-simulated radiation (SSR) used in sun protection factor (SPF) testing may influence the minimal erythema dose and SPF results further emphasizes the importance of global spectrum protection [33].
Fig. 3.
Protection profile of optimal daily UVR protection versus other day creams or sunscreens (all SPF 15), with various UVA1-PF, corresponding to various CW. Red line: day cream focused on UVB protection; yellow line: sunscreen with CW 360 nm; purple line: sunscreen with CW 370 nm; light blue line: optimal daily UV protection CW 380 nm; dark blue line: ideal sunscreen profile (protection by clothing and garments). Numbers in boxes stand for UVA1-PF. Logos stand for various UVA standards depending on countries [US FDA: US standards (CW = 370 nm only); EU: Europe (CW ≥ 370 nm and UVA-PF:SPF ratio > 0.33); AUS: transmission < 10% (320–360 nm); UK: United Kingdom (CW ≥ 370 nm and Boots 5-star rating UVA/UVB ≥ 0.9)]. Adapted from Osterwalder et al. [32] with permission. AUS Australia, CW critical wavelengths, EU European Union, FDA Food and Drug Administration, SPF sun protection factor, UV ultraviolet, UVA-PF ultraviolet A protection factor, UVA1-PF ultraviolet A1 protection factor, UVR ultraviolet radiation
Fig. 4.
Protection spectrum against solar radiation. IR infrared radiation, SPF sun protection factor, UVA-PF UVA protection factor
The concept of sunscreen homeostasis also considers the homeostasis with our environment. As shown by Ramos et al. [34], who reviewed the presence of organic UVR filters in wastewater treatment plants in Portugal, there is a need for the development of ecological solar UVR filters, based on naturally occurring filters. Natural compounds can offer photostable wide-spectrum filters, with reduced ecotoxicity and adverse effects [35]. Several species, including aquatic organisms, produce small molecules that protect them from the sun by absorbing harmful UVR, such as UV-absorbing mycosporine-like amino (MMA) acids [36].
Besides, it should be remembered that sunscreens do not address a homogeneous target population. Skins of different phototypes differ in their photobiological responses, including DNA repair, immunosuppression, apoptosis, anti-oxidative capabilities, and photoaging [37]. Differences in the melanocortin 1 receptor and in skin pigments are involved in the susceptibility to skin cancer [38]. Personalized photoprotection recommendations concerning skin cancer risk factors, desired treatment outcomes, health needs (e.g., vitamin D), and photoaging, based on the needs and preferences of the patient, are therefore essential [39].
Last but not least, on our way to sun–skin homeostasis, we should not forget that the most imponderable aspect is the human factor, based on beliefs, behaviors, and conduct. With individual exposure ranging from nearly total to minimal skin coverage, the challenge will surely require improved photoprotection strategies such as systemic photoprotectants and, ideally, universal photoeducation campaigns [40].
Acknowledgments
Special thanks to Dr Uli Osterwalder for his helpful discussions on homeostasis. We also thank Françoise Nourrit-Poirette and Marielle Romet (Santé Active Edition) who provided medical writing assistance on behalf of Laboratoires dermatologiques Avène.
Funding
Medical writing assistance was funded by Laboratoires dermatologiques Avène. Prof. Fernando Stengel received funds from Laboratoires dermatologiques Avène for traveling to and presenting at the conference.
Conflict of interest
Fernando Stengel declares that he has no conflicts of interest that might be relevant to the contents of this manuscript.
Disclosure statement
This article is published as part of a journal supplement wholly funded by Laboratoires dermatologiques Avène.
References
- 1.WHO. Global Solar UV Index: a practical guide. A joint recommendation of the World Health Organization, World Meteorological Organization, United Nations Environment Programme, and the International Commission on Non-Ionizing Radiation Protection. Geneva: WHO; 2002.
- 2.United Nations Environment Programme EEAP Environmental effects of ozone depletion and its interactions with climate change: progress report, 2016. Photochem Photobiol Sci. 2017;16(2):107–145. doi: 10.1039/C7PP90001E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupont E, Gomez J, Bilodeau D. Beyond UV radiation: a skin under challenge. Int J Cosmet Sci. 2013;35(3):224–232. doi: 10.1111/ics.12036. [DOI] [PubMed] [Google Scholar]
- 4.Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85(3):152–161. doi: 10.1016/j.jdermsci.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 5.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14(6):12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schalka S, Steiner D, Ravelli FN, Steiner T, Terena AC, Marcon CR, et al. Brazilian consensus on photoprotection. An Bras Dermatol. 2014;89(6 Suppl 1):1–74. doi: 10.1590/abd1806-4841.20143971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Wacker M, Holick MF. Sunlight and Vitamin D: a global perspective for health. Dermatoendocrinol. 2013;5(1):51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deluca HF. History of the discovery of vitamin D and its active metabolites. Bonekey Rep. 2014;8(3):479. doi: 10.1038/bonekey.2013.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naseem H, Wall AP, Sangster M, Paton RW. The presentation of rickets to orthopaedic clinics: return of the English disease. Acta Orthop Belg. 2011;77(2):239–245. [PubMed] [Google Scholar]
- 11.Kimlin M, Sun J, Sinclair C, Heward S, Hill J, Dunstone K, et al. Are the current Australian sun exposure guidelines effective in maintaining adequate levels of 25-hydroxyvitamin D? J Steroid Biochem Mol Biol. 2016;155(Pt B):264–270. doi: 10.1016/j.jsbmb.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Kockott D, Herzog B, Reichrath J, Keane K, Holick MF. New approach to develop optimized sunscreens that enable cutaneous vitamin D formation with minimal erythema risk. PLoS ONE. 2016;11(1):e0145509. doi: 10.1371/journal.pone.0145509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juzeniene A, Moan J. Beneficial effects of UV radiation other than via vitamin D production. Dermatoendocrinol. 2012;4(2):109–117. doi: 10.4161/derm.20013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin beta-endorphin mediates addiction to UV light. Cell. 2014;157(7):1527–1534. doi: 10.1016/j.cell.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Rhee H, Coebergh JW, de Vries E. Is prevention of cancer by sun exposure more than just the effect of vitamin D? A systematic review of epidemiological studies. Eur J Cancer. 2013;49(6):1422–1436. doi: 10.1016/j.ejca.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Weller RB. Sunlight has cardiovascular benefits independently of vitamin D. Blood Purif. 2016;41(1–3):130–134. doi: 10.1159/000441266. [DOI] [PubMed] [Google Scholar]
- 17.Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175(5):745–754. doi: 10.1001/jamainternmed.2015.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu D, Fernandez BO, Hamilton A, Lang NN, Gallagher JMC, Newby DE, et al. UVA irradiation of human skin vasodilates arterial vasculature and lowers blood pressure independently of nitric oxide synthase. J Invest Dermatol. 2014;134(7):1839–1846. doi: 10.1038/jid.2014.27. [DOI] [PubMed] [Google Scholar]
- 19.Oplander C, Volkmar CM, Paunel-Gorgulu A, van Faassen EE, Heiss C, Kelm M, et al. Whole body UVA irradiation lowers systemic blood pressure by release of nitric oxide from intracutaneous photolabile nitric oxide derivates. Circ Res. 2009;105(10):1031–1040. doi: 10.1161/CIRCRESAHA.109.207019. [DOI] [PubMed] [Google Scholar]
- 20.Feelisch M, Kolb-Bachofen V, Liu D, Lundberg JO, Revelo LP, Suschek CV, et al. Is sunlight good for our heart? Eur Heart J. 2010;31(9):1041–1045. doi: 10.1093/eurheartj/ehq069. [DOI] [PubMed] [Google Scholar]
- 21.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 22.Cowburn AS, Takeda N, Boutin AT, Kim JW, Sterling JC, Nakasaki M, et al. HIF isoforms in the skin differentially regulate systemic arterial pressure. Proc Natl Acad Sci USA. 2013;110(43):17570–17575. doi: 10.1073/pnas.1306942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halliday GM, Byrne SN. An unexpected role: UVA-induced release of nitric oxide from skin may have unexpected health benefits. J Invest Dermatol. 2014;134(7):1791–1794. doi: 10.1038/jid.2014.33. [DOI] [PubMed] [Google Scholar]
- 24.Murray CJ, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380(9859):2063–2066. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RS, Titze J, Weller R. Cutaneous control of blood pressure. Curr Opin Nephrol Hypertens. 2016;25(1):11–15. doi: 10.1097/MNH.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahmoud BH, Ruvolo E, Hexsel CL, Liu Y, Owen MR, Kollias N, et al. Impact of long-wavelength UVA and visible light on melanocompetent skin. J Invest Dermatol. 2010;130(8):2092–2097. doi: 10.1038/jid.2010.95. [DOI] [PubMed] [Google Scholar]
- 27.Kohli I, Chaowattanapanit S, Mohammad TF, Nicholson CL, Fatima S, Jacobsen G, et al. Synergistic effects of long wavelength ultraviolet al and visible light on pigmentation and erythema. Br J Dermatol. 2018;178(5):1173–1180. doi: 10.1111/bjd.15940. [DOI] [PubMed] [Google Scholar]
- 28.Diffey B, Cadars B. An appraisal of the need for infrared radiation protection in sunscreens. Photochem Photobiol Sci. 2016;15(3):361–364. doi: 10.1039/C5PP00451A. [DOI] [PubMed] [Google Scholar]
- 29.Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J Mol Cell Cardiol. 2009;47(2):256–263. doi: 10.1016/j.yjmcc.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piazena H, Kelleher DK. Effects of infrared-A irradiation on skin: discrepancies in published data highlight the need for an exact consideration of physical and photobiological laws and appropriate experimental settings. Photochem Photobiol. 2010;86(3):687–705. doi: 10.1111/j.1751-1097.2010.00729.x. [DOI] [PubMed] [Google Scholar]
- 31.Osterwalder U, Hareng L. Global UV filters: current technologies and future innovations. In: Wang S, Lim H, editors. Principles and practice of photoprotection. Cham: Adis; 2016. [Google Scholar]
- 32.Osterwalder U, Herzog B, Wang SQ. Advance in sunscreens to prevent skin cancer. Expert Rev Dermatol. 2011;6(5):479–491. doi: 10.1586/edm.11.50. [DOI] [Google Scholar]
- 33.Brandt M, Rohr M, Schrader A. Influence of VIS/NIR radiation on the characteristics of sunscreen and human skin. IFSCC Magazine. 2001;4(1):15–19. [Google Scholar]
- 34.Ramos S, Homem V, Alves A, Santos L. A review of organic UV-filters in wastewater treatment plants. Environ Int. 2016;86:24–44. doi: 10.1016/j.envint.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence KP, Long PF, Young AR. Mycosporine-like amino acids for skin photoprotection. Curr Med Chem. Epub. 2017 doi: 10.2174/0929867324666170529124237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shick JM, Dunlap WC. Mycosporine-like amino acids and related Gadusols: biosynthesis, acumulation, and UV-protective functions in aquatic organisms. Annu Rev Physiol. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- 37.Fajuyigbe D, Young AR. The impact of skin colour on human photobiological responses. Pigment Cell Melanoma Res. 2016;29(6):607–618. doi: 10.1111/pcmr.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasti TH, Timares L. MC1R, eumelanin and pheomelanin: their role in determining the susceptibility to skin cancer. Photochem Photobiol. 2015;91(1):188–200. doi: 10.1111/php.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cestari T, Buster K. Photoprotection in specific populations: children and people of color. J Am Acad Dermatol. 2017;76(3S1):S110–S121. doi: 10.1016/j.jaad.2016.09.039. [DOI] [PubMed] [Google Scholar]
- 40.Stengel FM, Fernandez JF. Education and behavioral change for sun protection. J Cosmet Dermatol. 2005;4(2):83–88. doi: 10.1111/j.1473-2165.2005.40206.x. [DOI] [PubMed] [Google Scholar]
- 41.Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165–1168. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- 42.Parrish JA, Jaenicke KF, Anderson RR. Erythema and melanogenesis action spectra of normal human skin. Photochem Photobiol. 1982;36(2):187–191. doi: 10.1111/j.1751-1097.1982.tb04362.x. [DOI] [PubMed] [Google Scholar]
- 43.de Gruijl FR. Skin cancer and solar UV radiation. Eur J Cancer. 1999;35(14):2003–2009. doi: 10.1016/S0959-8049(99)00283-X. [DOI] [PubMed] [Google Scholar]