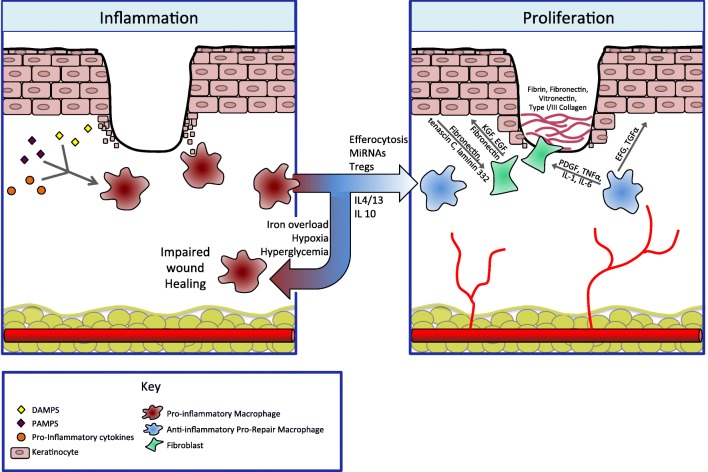
Fig. 2.
Legend: the transition from pro-inflammatory macrophages to anti-inflammatory macrophages is a key regulatory step, allowing the immune system to promote both ECM formation and re-epithelialization. During the inflammatory phase, pro-inflammatory macrophages dominate. They are activated by danger signals such as pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) as well as pro-inflammatory cytokines. This phenotype is responsible for clearance of debris and prevention of infection. Persistence of inflammation results in a non-healing wound. Normally, macrophages transition to an anti-inflammatory phenotype in response to signals such as neutrophil apoptosis and engulfment (efferocytosis) as well as other local immune signals. This transition is inhibited in the setting of iron overload, hypoxia, and hyperglycemia. These pro-healing, anti-inflammatory macrophages are responsible for resolution of tissue inflammation and contribute to angiogenesis and tissue repair. During the proliferative phase, new blood vessels and granulation tissue are laid down and keratinocytes re-epithelialize. Pro-repair macrophages send signals to both fibroblasts and keratinocytes themselves. To keratinocytes, they release epidermal growth factor (EGF) and transforming growth factor-α (TGF-α), which drive keratinocyte proliferation and migration. Through platelet-derived growth factor (PDGF), TNF-α, IL-1, and IL-6, pro-repair macrophages signal fibroblasts to lay down granulation tissue, comprised of fibrin, fibronectin, as well as collagen. In turn, fibroblasts further stimulate keratinocyte proliferation and migration through keratinocyte growth factor (KGF), EGF, and fibronectin. Keratinocytes themselves also activate fibroblasts in a feedback loop through the production of fibronectin, tenascin C, and laminin 332