Abstract
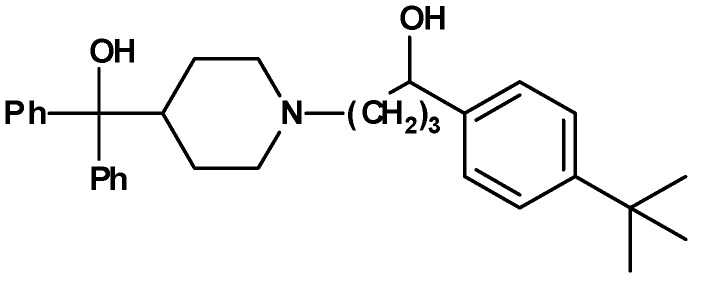
Terfenadine (4-[4-(hydroxydiphenylmethyl)-1-piperidyl]-1-(4-tert-butyl-phenyl)-butan-1-ol) was identified in a biological screening to be a moderate inhibitor (27 % inhibition) of the CD81-LEL–HCV-E2 interaction. To increase the observed biological activity, 63 terfenadine derivates were synthesized via microwave assisted nucleophilic substitution. The prepared compounds were tested for their inhibitory potency by means of a fluorescence labeled antibody assay using HUH7.5 cells. Distinct structure-activity relationships could be derived. Optimization was successful, leading to 3g, identfied as the most potent compound (69 % inhibition). Experiments with viral particles revealed that there might be additional HCV infection reducing mechanisms.
Keywords: Hepatitis C Virus, CD81-receptor, large extracellular loop, terfenadine derivatives, microwave assisted syntheses
Introduction
Despite the discovery of the Hepatitis C Virus (HCV) more than 15 years ago, chronic HCV infection is still incurable in many patients, leading to cirrhosis, end-stage liver disease and hepatocellular carcinoma [1]. According to the 2002 WHO report, in 2001 more than 280,000 deaths worldwide were attributable to HCV infection [2]. The large extracellular loop (LEL) of the human CD81 cell surface protein, a member of the tetraspanin family, was identified as a binding partner for the Hepatitis C Virus envelope glycoprotein E2 (HCV-E2) [3]. Since inhibition of this interaction prevents HCV from infecting hepatocytes, the HCV principal target cells [3], the aim of the present work was to prepare compounds which restrain the CD81-LEL–HCV-E2 interaction by binding to the LEL. Another approach to inhibit this interaction using compounds that bind to the E2 glycoprotein of HCV was recently published by Van Compernolle et al. [3].
Results and Discussion
Biological Screening
The starting point of this work was a biological screening of natural products, current drugs and our in-house substance library (approximately 350 compounds, including several structurally different antihistamines) using a medium throughput assay developed in our group [4]. This assay is based on a procedure developed by Pileri et al. [5], in which the compounds inhibit the binding of the fluorescence-labeled CD81 antibody JS81 to HUH7.5 cells.
As an outcome of this screening an antihistamine,, terfenadine (Figure 1), was found to be a moderate inhibitor of the CD81-LEL–HCV-E2 interaction (27 % at 50 µM), whereas all other antihistamines showed no biological activity.
Figure 1.
Terfenadine.
Structure Modification
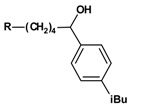
Based on these results, a series of terfenadine derivatives was prepared seeking to increase the inhibitory activity and to derive structure-activity relationships. The following structural features were modified: length of the alkyl “linker” between the piperidine and the phenyl moiety, alkyl substituent on the phenyl ring, secondary hydroxy group and the azacyclonol moiety.
Syntheses
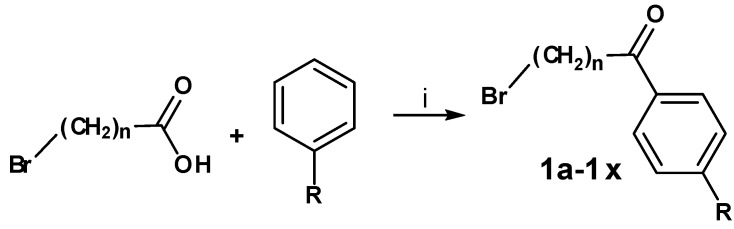
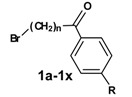
The first step of the preparation was a Friedel-Crafts (FC) acylation of the benzene derivative with aluminium chloride as catalyst and the carboxylic acid chloride of the corresponding ω-bromo-carboxylic acid, prepared using thionyl chloride (Scheme 1). The synthesized 1-aryl-ω-bromo ketones are shown in Table 1.
Scheme 1.
Reagents and conditions for the FC acylation and synthesized 1-aryl-ω-bromo ketones.
Reagents and conditions: (i) 1. SOCl2, rt, 30 min 2. dry CH2Cl2, AlCl3, 0 °C, 45 min (n = 3-5; R = H; C1-C4).
Table 1.
Synthesized 1-aryl-ω-bromo ketones 1a-1x (n = 3-5; R = H; C1-C4).
| ||||||
| R | n | compound | n | compound | n | compound |
| H | 5 | 1a | 4 | 1i | 3 | 1q |
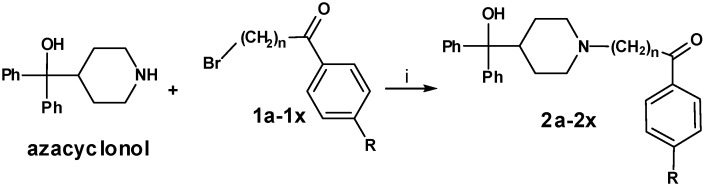
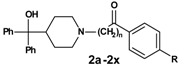
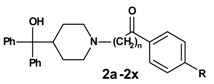
In the second reaction step (Scheme 2) the precursors 1a-1x were coupled to azacyclonol according to a described procedure [6]. The classical nucleophilic substitution was optimized for microwave assisted synthesis. The desired compounds were obtained in satisfying yields in a very short time (5-45 minutes). This led to compounds 2a-2x, shown in Table 2.
Scheme 2.
Reagents and conditions: (i) K2CO3, catalytic KI and 18-crown-6, dry acetonitrile, microwave: 150 Watt, 6.5 bar, 175 °C, 45 min (100 Watt, 140 °C, 3 h: 2g, 2h, 2t, 2u, 2v, 2w), n = 3-5; R = H; C1-C4.
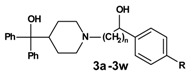
Table 2.
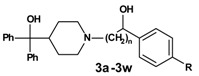
Synthesized terfenadine derivatives 2a-2x and 3a-3w (n = 3-5; R = H; C1-C4).
|
|
||||||||||||
| R | n | compound | n | compound | n | compound | n | compound | n | compound | n | compound | |
| H | 5 | 2a | 4 | 2i | 3 | 2q | 5 | 3a | 4 | 3i | 3 | 3q | |
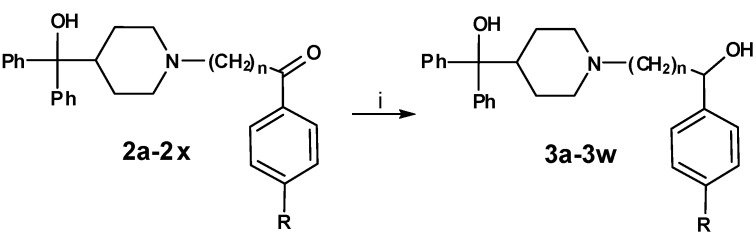
A sodium borohydride reduction (Scheme 3) of the ketone function followed. The prepared alcohols 3a-3w, which were obtained as racemates, are also listed in Table 2.
Scheme 3.
Reagents and conditions: (i) NaBH4, MeOH, 0 °C, 1 h (n = 3-5; R = H; C1-C4).
Since azacyclonol, the second component for the coupling reaction, was commercially available, this synthetic procedure facilitated the preparation of a large variety of compounds in a minimum amount of time. For further modification of the secondary hydroxy group the ester 5a, the amide 5b and the alkane 8a were synthesized.
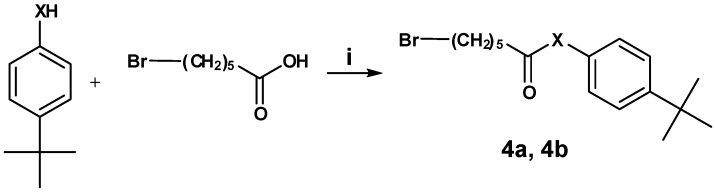
Activation of 6-bromo hexanoic acid with thionyl chloride followed by addition of commercially available 4-tert-butyl phenol and 4-tert-butyl aniline, respectively, led to the ω-bromo substituted precursors 4a and 4b (Scheme 4).
Scheme 4.
Reagents and conditions: (i) 1. SOCl2, rt, 1 h 2. NEt3, dry CH2Cl2, 0 °C 30 min, rt 1 h (X = -O-: 4a, X = -NH-: 4b).
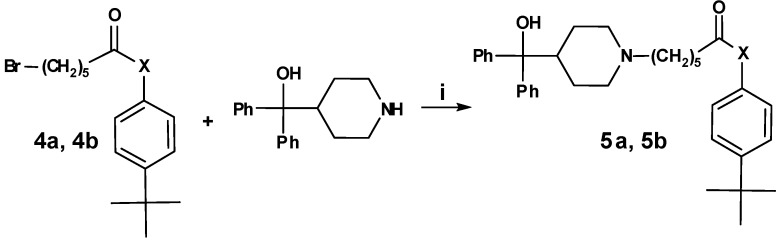
Next, these precursors were nucleophilically coupled to azacyclonol, leading to compounds 5a and 5b, respectively (Scheme 5).
Scheme 5.
Reagents and conditions: (i) K2CO3, catalytic KI and 18-crown-6, dry acetonitrile, 150 Watt, 140 °C, 1 h (X = -O-: 5a, X = -NH-: 5b).
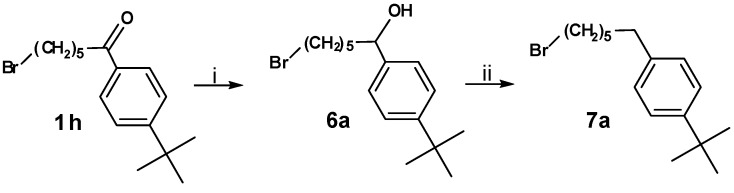
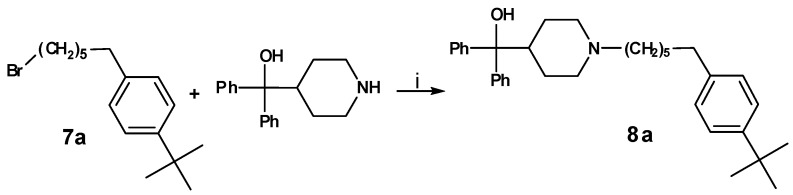
For the synthesis of 8a compound 1h was reduced using sodium borohydride. The resulting alcohol 6a was converted into the corresponding alkane 7a by means of a mixture of indium(III) chloride and chlorodiphenylsilane (Scheme 6) and subsequently coupled to azacyclonol, leading to compound 8a (Scheme 7).
Scheme 6.
Reagents and conditions: (i) see Scheme 3; (ii) indium(III) chloride, chlorodiphenylsilane, dry dichloromethane, 3 h, rt.
Scheme 7.
Reagents and conditions: (i) K2CO3, catalytic KI and 18-crown-6, dry acetonitrile, 50 Watt, 100 °C, 5 min
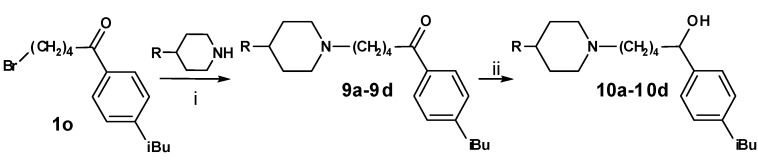
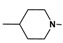
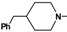
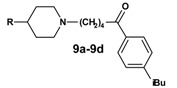
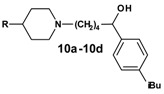
The exchange of the azacyclonol component by smaller piperidine moieties started from the 1-aryl-ω-bromo ketone 1o and was accomplished via microwave assisted nucleophilic substitution, followed by reduction of the ketone (Scheme 8). The prepared compounds 9a-9d, as well as the corresponding alcohols 10a-10d, obtained as racemates, are shown in Table 3.
Scheme 8.
Reagents and conditions: (i) K2CO3, catalytic KI and 18-crown-6, dry acetonitrile, microwave 150 Watt, 6.5 bar, 175 °C, 45 min; (ii) NaBH4, MeOH, 0 °C, 1 h (R = -H, -CH3, -OH, benzyl).
Table 3.
Synthesized terfenadine derivatives 9a-9d and 10a-10d.
|
|
||
| R | compound | R | compound |
| H | 9a | H | 10a |
| Methyl | 9b | Methyl | 10b |
| Hydroxyl | 9c | Hydroxyl | 10c |
| Benzyl | 9d | Benzyl | 10d |
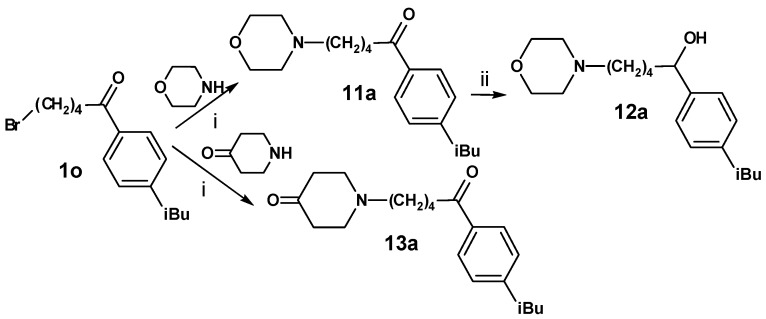
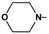
Furthermore, 1o was reacted with morpholine and piperidin-4-one resulting in compounds 11a, 12a and 13a (scheme 9). The morpholine derivative 12a was obtained as a racemate.
Scheme 9.
Reagents and conditions: (i) K2CO3, catalytic KI and 18-crown-6, dry acetonitrile, microwave 150 Watt, 6.5 bar, 175 °C, 45 min; (ii) NaBH4, MeOH, 0 °C, 1 h.
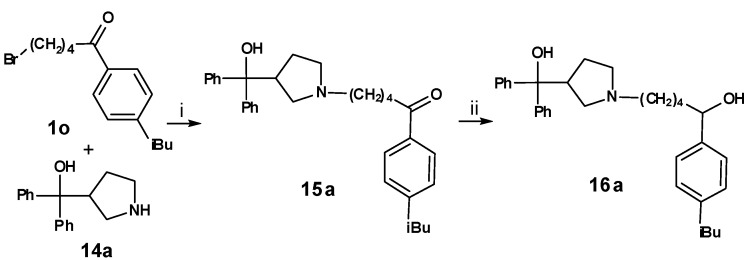
For the syntheses of the pyrrolidine compounds 15a and 16a, 1o was coupled with diphenyl(pyrrolidin-3-yl)methanol (14a). The resulting ketone 15a was reduced with sodium borohydride (Scheme 10), leading to the desired compound as a racemate.
Scheme 10.
Reagents and conditions: (i) K2CO3, catalytic KI and 18-crown-6, dry acetonitrile, microwave 150 Watt, 6.5 bar, 175 °C, 45 min; (ii) NaBH4, MeOH, 0 °C, 1 h.
Biological evaluation
The synthesized terfenadine derivatives were tested for their inhibitory potency using the antibody neutralization (AN) assay mentioned above (Table 4, Table 5, Table 6, Table 7 and Table 8) [4]. In this assay the potential inhibitors and the fluorescence-labeled antibody JS81 compete for binding to the LEL on the CD81 receptor molecule. The reduction of the interaction of JS81 with CD81 on HUH7.5 cells caused by the compounds decreases fluorescence which is measured by FACS in comparison to untreated control cells.
Table 4.
Inhibition of protein interaction in AN assay by compounds 2a-2x (concentration: 50 µM, standard deviation ≤ 6 %).
| ||||||
| n = 5 | n = 4 | n = 3 | ||||
| R | compound | inhibition (%) | compound | inhibition (%) | compound | inhibition (%) |
| H | 2a | 10 | 2i | 4 | 2q | 7 |
Table 5.
Inhibition of protein interaction in AN assay by compounds 3a-3w (concentration: 50 µM, standard deviation ≤ 6 %).
| ||||||
| n = 5 | n = 4 | n = 3 | ||||
| R | compound | inhibition (%) | compound | inhibition (%) | compound | inhibition (%) |
| H | 3a | 4 | 3i | 1 | 3q | 5 |
Table 6.
Inhibition of protein interaction in AN assay by compounds 9a-13a compared to compounds 2o and 3o (concentration: 50 µM, standard deviation ≤ 6 %).
|
|
|||
| R | compound | inhibition (%) | compound | inhibition (%) |
| azacyclonol | 2o | 60 | 3o | 67 |
Table 7.
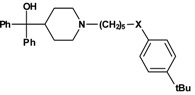
Inhibition of protein interaction in AN assay by compounds 5a-5b, 8a compared to compounds 2h and 3h (R = azacyclonol, concentration: 50 µM, standard deviation ≤ 6 %).
| |||||
| X | compound | inhibition (%) | X | compound | inhibition (%) |
|
3h | 58 |
|
8a | 9 |
Table 8.
Inhibition of protein interaction in AN assay by compounds 15a, 16a compared to compounds 2p and 3p (R = azacyclonol, concentration: 50 µM, standard deviation ≤ 6 %).
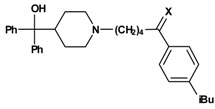
|
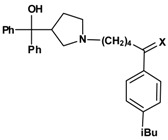
|
|||
| X | compound | inhibition (%) | compound | inhibition (%) |
| O | 2o | 60 | 15a | 64 |
The % inhibition values of compounds 2a-2x (Table 4) and 3a-3w (Table 5) show that the alkyl substituent R on the phenyl ring has a major influence on the activity. Another important feature is obviously the length of the linker, whereas the activities of the ketones (Table 4) and the corresponding alcohols (Table 5) do not differ significantly, at least in case of the n-propyl and n-butyl compounds.
Increasing the size of R from hydrogen to bulkier alkyl substituents increases inhibition. A maximum inhibition, not depending on the linker length, could be reached by the use of n-propyl for the ketones and iso-butyl for the alcohols, with the exception of compounds 2d and 3w, which are less active than the analogous 2e and 3v. Generally, compounds with n = 3 show a lower inhibition than those with n = 4 or 5, with the exception of 3v, which is more active than 3f and 3n. Furthermore, reduction of the ketones 2a-2x to the corresponding alcohols 3a-3w and terfenadine led to a decreased activity for compounds with small substituents at the phenyl ring, whereas a reduction of bulky substituted compounds led to derivatives with comparable inhibition. The reduction of the ketones with R = iso-propyl to the corresponding alcohols led to a nearly complete loss of biological activity. The most active compound in this series of terfenadine derivatives is 3g (69 % inhibition). Therefore, the hexanol linker combined with an iso-butyl group at the phenyl ring is the most favorable substitution pattern in this class of compounds.
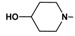
Replacement of the azacyclonol moiety by smaller piperidine residues (9a-9c, 10a-10c and 11a-13a) led to a loss of inhibitory activity compared to the reference compounds 2o and 3o (Table 6). Substitution of the piperidine group by a 4-benzyl group (9d and 10d) increased the inhibition to a moderate level. Again bulky substituents are essential for activity at this part of the molecule.
Reduction of the secondary hydroxy group of 3h to the corresponding alkane 8a led to a strong decrease of inhibition (Table 7). Exchange of the ketone of 2h by an ester function (compound 5a) did not influence the inhibitory activity, whereas an amide group (compound 5b) increased the inhibition. These SAR results indicate that the functional group X might act as an H-bond acceptor interacting with the CD81 protein.
Exchange of the piperidine of the azacyclonol moiety of 2o and 3o by pyrrolidine (compounds 15a and 16a) did not lead to a significant change of the inhibitory activity (Table 8). Obviously due to the flexibility of the chain, appropriate conformations can be found for both heterocycles.
Selected compounds with high, moderate and low inhibition in the AN assay were tested for their inhibition in an infectivity assay developed by Pietschmann and coworkers [7] (Table 9), in which HUH7.5 cells were incubated with HCV particles of the LUC-Jc1 genome (genotype 2a) [8] and potential inhibitors. After 48 hours, the cells were lysed and luciferase activity (a marker of infection and viral replication) was measured. The infectivity assay was performed at the highest non-cytotoxic concentrations of the compounds (0.5 µM: 2e, 2s, 3f, 3g, 3i, 8a and 5 µM: 3q, 10d).
Table 9.
Inhibition of infectivity of viral particles by selected compounds.
| Compound | Antibody neutralization assay (inhibition (%), 50 µM) | Infectivity assay with viral particles (inhibition (%), 0.5 µM) |
|---|---|---|
| 2e | 63 | 43 |
Concentration: 0.5 µM, a 5 µM, standard deviation ≤ 14 % compared to AN assay data.
Compounds with high biological activity in the neutralization assay showed good inhibition in the infectivity assay as well. This clearly indicates that inhibition of the protein-protein interaction leads to a reduction of infectivity. On the other hand there are compounds which reduced infectivity without having been active in the AN assay. One plausible reason for this phenomenon could be an interaction of these compounds with an additional target which is involved in viral infection.
Conclusions
After having identified terfenadine as an active compound inhibiting the CD81-LEL–HCV-E2 interaction by 27 %, the compound was structurally modified with the aim of increasing the activity. The results obtained clearly demonstrate that a bulky substituent at the phenyl moiety as well as an additional bulky substituent at the other part of the molecule (like azacyclonol) are necessary for activity as well as the secondary OH-group. Another H-bond acceptor like ketone, ester or amide can replace the latter. Importantly, the activity of the parent compound can be increased by elongation of the alkyl chain resulting in 3g with an inhibition of 69 %. A further experiment with viral particles and HUH7.5 cells showed that there are possibly further mechanisms by which the test compounds unfold their anti-viral activity. These mechanisms remain to be elucidated.
Experimental
General
Solvents and reagents were used as received from commercial distributors without further purification. Anhydrous reactions were conducted under a nitrogen atmosphere. Proton and carbon NMR spectra (in CDCl3, unless otherwise noted) were recorded on a Bruker AM 500 instrument The proton NMR spectra were recorded at 500 MHz, the carbon NMR spectra at 125 MHz. Chemical shifts δ are reported in ppm units. Molecular mass was determined by liquid chromatography – tandem mass spectrometry (LC-MS/MS) using a TSQ Quantum from Thermo Finnigan equipped with an electro spray interface and connected to a Surveyor HPLC (Thermo Finnigan). Positive and negative ion mass spectra were recorded (mass range m/z 150–1500) in normal scan mode. Melting points were determined using a Stuart Scientific SMP3 melting point apparatus. IR measurements were performed on a Bruker Vector 33 at a frequency range from 4000–250 cm-1. Wave numbers υ are reported in cm-1. Microwave assisted syntheses was performed using a CEM DISCOVER microwave oven or a MLS MultiSYNTH microwave oven respectively. Preparative HPLC was carried out using an Agilent Technologies, 1200 Series Isocratic, with an Agilent Prep-C18 (5 µm; 30x100mm) preparative column using 70:30 methanol/water as eluent. Flash chromatography was performed using Merck silica gel 35/40–63/70.
Antibody neutralization assay.
For the antibody neutralization assay HUH7.5 cells were used, derived from a human hepatoma cell line which was preselected via FACS for cells displaying high amounts of CD81 on their cell surface. Cells were grown under standard conditions (Dulbecco’s Modified Eagle’s Medium with 10 % FCS, 1% penicillin/streptavidin, pH 7.4, at 37°C with 5% CO2). 1∙105 cells were incubated with 100 µL (50µM) of the potential inhibitor. The inhibitor solution contained 1 % DMSO, a concentration tolerated by the used HUH7.5 cells and without influence on MFI (mean fluorescence intensity) values (data not shown). Incubation was performed in 96 transwell plates for 10 minutes at room temperature. Subsequently 4 µL of the fluorescence-labeled anti human CD81 antibody JS81 (Pharmingen) and 21 µL of phosphate buffered saline buffer (PBS) were added and kept at room temperature for 10 minutes. The final concentration of JS81 in the assay was 1.6∙10-3 µg/µL. After addition of 125 µL of PBS the cell suspension was incubated in the dark for 5 hours followed by FACS (Becton Dickison – FACS Calibur; software: Cell Quest Pro) analysis. Ten thousand vital cells out of a predetermined area were analyzed for their MFI.
Infectivity assay.
In vitro synthesis of the LUC-Jc1 RNA genome in which the luciferase reporter gene was inserted into the genome of the Jc1 HCV variant and electroporation of Huh-7 cells was performed as described previously [9, 10]. We collected culture medium containing viral particles 48 h after transfection. HUH7.5 target cells were seeded 24 h before infection at a density of 6∙105 cells/well in a 24 well plate. Cells were infected with 200 µL inoculum containing the potential inhibitors at 5 µM or 0.5 µM for 4 h. Concentrations were chosen according to the highest non-cytotoxic concentration of the given compound. Cells were washed, complete medium was added and cells were cultured for 48 h. Afterwards cells were lysed for luciferase assay as previously described [7].
Chemistry
General procedure for the Friedel-Crafts-acylation
The carboxylic acid was stirred with thionyl chloride (10 mL) at room temperature for 1h. After removing the excess of thionyl chloride under reduced pressure, dry dichloromethane (15 mL) and the benzene derivative (1 equivalent, referring to the carboxylic acid) were added. The solution was cooled to 0 °C and AlCl3 (1.2 equivalents) were added. After stirring at that temperature for 45 min, the mixture was hydrolyzed, the layers separated and the water phase was extracted with dichloromethane two times. The combined organic layers were dried and the solvent removed. Flash chromatography using 1/15 ethyl acetate/n-hexane as eluent led to the desired compounds.
General procedure for the nucleophilic substitution
Azacyclonol (1 equivalent), ketone (1 equivalent), potassium carbonate (5 equivalents), a catalytic amount of potassium iodide and 18-crown-6 were stirred in dry acetonitrile (4 mL) in the microwave (CEM Discover) at 150 Watt, 6.5 bar, 175 °C for 45 min or (MultiSYNTH) at 100 Watt, 140 °C, 3 h (2g, 2h, 2t, 2u, 2v, 2w) or at 50 Watt, 100 °C, 5 min (8a). The microwave assisted reactions were performed in a continuous air stream cooled closed vessel. Reaction temperature was monitored via an IR sensor in both microwave ovens and an additional fiber optic component in case of the MultiSYNTH. After filtering off the remaining precipitate the solvent was removed. The impurities could not be removed completely by flash chromatography (6/1 ethyl acetate/n-hexane + 3 % NEt3), so preparative HPLC (isocratic 70/30 MeOH/H2O) was performed and the pure product isolated.
General procedure for the reduction of the ketones
Sodium borohydride (5 equivalents) and ketone (1 equivalent) were added to 2 mL methanol at 0 °C. After stirring for 30 min a spatula tip of sodium borohydride was added to the mixture which was stirred for another 30 min at 0 °C. The mixture was hydrolyzed using 2 mL NH4Cl-solution. After removing the solvents under reduced pressure, the remaining solid was washed two times with methanol and ethyl acetate (2 mL each). The combined organic layers were freed from solvent and the raw product was purified by preparative HPLC (isocratic MeOH/H2O: 70/30) to give the racemates in satisfying yields.
General procedure for the ester and amide coupling
The carboxylic acid (1 equivalent) was stirred with an excess of thionyl chloride for 1 hour at room temperature. The resulting clear solution was freed from remaining thionyl chloride under reduced pressure. The acid chloride was dissolved in dry dichloromethane and added dropwise at 0 °C to a solution of the corresponding alcohol or amine (1.2 equivalents) and an equimolar amount of triethylamine in dry dichloromethane. After stirring for 30 minutes at 0 °C the mixture was warmed to room temperature and stirred for 1 hour. The precipitated solid was filtered off. The solvent was removed and the raw product purified by column flash chromatography using an ethyl acetate/n-hexane mixture.
6-Bromo-1-phenylhexan-1-one (1a). Yield: 26 %; 1H-NMR: 7.88–7.85 (2 H, m), 7.49–7.45 (1 H, m), 7.39–7.35 (2 H, m), 3.35–3.32 (2 H, m), 2.92–2.88 (2 H, m), 1.86–1.79 (2 H, m), 1.72–1.64 (2 H, m), 1.48–1.40 (2 H, m) [Lit. [11] 400 MHz, CDCl3: 7.94 (2 H, m), 7.55 (1 H, m), 7.45 (2 H, m), 3.41 (2 H, t, J = 6.80), 2.98 (2 H, t, J = 7.00), 1.90 (2 H, m), 1.76 (2 H, m), 1.53 (2 H, m)]; 13C-NMR: 199.96, 136.95, 133.02, 128.61, 128.02, 38.29, 33.69, 32.66, 27.89, 23.33.
6-Bromo-1-(4-methylphenyl)hexan-1-one (1b). Yield: 50 %; 1H-NMR: 7.76 (2 H, d, J = 8.06), 7.16 (2 H, d, J = 7.98), 3.34–3.31 (2 H, t, J = 6.78), 2.88–2.84 (2 H, t, J = 7.44), 2.31 (3 H, s), 1.85–1.78 (2 H, m), 1.70–1.62 (2 H, m), 1.47–1.39 (2 H, m) [Lit. [12] CDCl3: 7.85 (2 H, d, J = 7.30), 7.25 (2 H, d, J = 8.60), 3.42 (2 H, d, J = 8.60), 2.96 (2 H, t, J = 7.30), 2,41 (3 H, s), 1.97–1.86 (2 H, m), 1.82–1.74 (2 H, m), 1.58–1.47 (2 H, m)]; 13C-NMR: 199.61, 143.72, 134.47, 129.26, 128.13, 38.14, 33.67, 32.65, 27.89, 23.41, 21.62.
6-Bromo-1-(4-ethylphenyl)hexan-1-one (1c). Yield: 75 %; 1H-NMR: 7.89 (2 H, d, J = 8.34), 7.29 (2 H, d, J = 8.49), 3.43 (2 H, t, J = 6.79), 2.99–2.96 (2 H, m), 2.74–2.68 (2 H, m), 1.95–1.88 (2 H, m), 1.81–1.73 (2 H, m), 1.57–1.50 (2 H, m), 1.26 (3 H, t, J = 7.60) [Lit. [12] CDCl3: 7.88 (1 H, d, J = 8.60), 7.28 (1 H, d, J = 8.60), 3.43 (2 H, t, J = 7.00), 2.97 (2 H, t, J = 7.30), 2.71 (2 H, q, J = 7.20), 2.04–1.48 (6 H, m), 1.26 (3 H, t, J = 7.60)]; 13C-NMR: 199.65, 148.91, 133.65, 127.22, 127.06, 37.16, 32.67, 31.64, 27.91, 26.89, 22.41, 14.22.
6-Bromo-1-(4-n-propylphenyl)hexan-1-one (1d). Yield: 74 %; 1H-NMR: 7.84 (2 H, d, J = 8.32), 7.23 (2 H, d, J = 8.35), 3.40 (2 H, t, J = 6.78), 2.94 (2 H, t, J = 7.24), 2.63–2.60 (2 H, m), 1.92–1.86 (2 H, m), 1.77–1.71 (2 H, m), 1.67–1.60 (2 H, m), 1.53–1.47 (2 H, m), 0.92 (3 H, t, J = 7.34); 13C-NMR: 199.69, 148.45, 134.75, 128.71, 128.17, 38.20, 38.04, 33.69, 32.70, 27.95, 24.26, 23.46, 13.81.
6-Bromo-1-(4-iso-propylphenyl)hexan-1-one (1e). Yield: 65 %; 1H-NMR: 7.89 (2 H, d, J = 8.40), 7.30 (2 H, d, J = 8.14), 3.42 (2 H, t, J = 6.79), 2.99–2.93 (3 H, m), 1.94–1.88 (2 H, m), 1.79–1.73 (2 H, m), 1.56–1.49 (2 H, m), 1.26 (6 H, d, J = 6.93); 13C-NMR: 199.66, 154.50, 134.88, 128.31, 126.70, 38.21, 34.27, 33.69, 32.70, 27.96, 23.72. 23.47.
6-Bromo-1-(4-n-butylphenyl)hexan-1-one (1f). Yield: 44 %; 1H-NMR: 7.98 (2 H, d, J = 8.25), 7.37 (2 H, d, J = 8.03), 3.53 (2 H, t, J = 6.77), 3.09–3.06 (2 H, m), 2.79–2.76 (2 H, m), 2.05–2.00 (2 H, m), 1.91–1.85 (2 H, m), 1.76–1.70 (2 H, m), 1.67–1.61 (2 H, m), 1.51–1.43 (2 H, m), 1.04 (3 H, t, J = 7.32); 13C-NMR: 199.66, 148.69, 134.71, 128.66, 128.19, 38.20, 35.71, 33.68, 33.29, 32.70, 27.95, 23.46, 22.36, 13.95.
6-Bromo-1-(4-iso-butylphenyl)hexan-1-one (1g). Yield: 68 %; 1H-NMR: 7.89 (2 H, d, J = 8.28), 7.23 (2 H, d, J = 8.28), 3.43 (2 H, t, J = 6.79), 2.97 (2 H, t, J = 7.35), 2.21 (2 H, d, J = 7.21). 1.95–1.88 (3 H, m), 1.81–1.75 (2 H, m), 1.57–1.51 (2 H, m), 0.92 (6 H, t, J = 6.62); 13C-NMR: 199.64, 147.44, 134.79, 129.31, 128.01, 45.38, 38.17, 33.61, 32.68, 30.11, 27.93, 23.43, 22.35.
6-Bromo-1-(4-tert-butylphenyl)hexan-1-one (1h). Yield: 60 %; 1H-NMR: 7.89 (2 H, d, J = 8.50), 7.47 (2 H, d, J = 8.52), 3.41 (2 H, t, J = 6.79), 2.96 (2 H, t, J = 7.35), 1.94–1.88 (2 H, m), 1.79–1.73 (2 H, m), 1.56–1.50 (2 H, m), 1.34 (9 H, s); 13C-NMR: 199.60, 156.68, 134.44, 128.00, 125.53, 38.18, 35.10, 33.62, 32.68, 31.12, 27.94, 23.46.
5-Bromo-1-phenylpentan-1-one (1i). Yield: 11 %; 1H-NMR: 7.96–7.94 (2 H, m), 7.58–7.54 (1 H, m), 7.48–7.45 (2 H, m), 3.38 (2 H, t, J = 6.62), 3.03–3.00 (2 H, m), 1.99–1.87 (4 H, m) [Lit. [13] CDCl3: 7.90–7.10 (5 H, m), 3.40 (2 H, t), 2.99 (2 H, t), 1.91 (4 H, m)]; 13C-NMR: 199.53, 136.80, 133.05, 128.59, 127.97, 37.38, 33.29, 32.16, 22.72.
5-Bromo-1-(4-methylphenyl)pentan-1-one (1j). Yield: 57 %; 1H-NMR: 7.83 (2 H, d, J = 8.20), 7.23 (2 H, d, J = 7.88), 3.43–3.41 (2 H, m), 2.97–2.94 (2 H, m), 2.38 (3 H, s), 1.94–1.84 (4 H, m); 13C-NMR: 199.16, 143.83, 134.38, 129.29, 128.13, 37.29, 33.41, 32.25, 22.86, 21.63.
5-Bromo-1-(4-ethylphenyl)pentan-1-one (1k). Yield: 68 %; 1H-NMR: 7.78 (2 H, d, J = 8.03), 7.17 (2 H, d, J = 8.03), 3.34–3.31 (2 H, m), 2.88–2.85 (2 H, m), 2.62–2.57 (2 H, m), 1.84–1.76 (4 H, m), 1.15 (3 H, t, J = 7.53); 13C-NMR: 199.19, 150.02, 134.66, 128.29, 128.15, 37.35, 33.39, 32.32, 28.98, 22.93, 15.25.
5-Bromo-1-(4-n-propylphenyl)pentan-1-one (1l). Yield: 55 %; 1H-NMR: 7.87 (2 H, d, J = 8.33), 7.26 (2 H, d, J = 8.45), 3.46–3.43 (2 H, m), 3.00–2.97 (2 H, m), 2.66–2.62 (2 H, m), 1.99–1.85 (4 H, m), 1.71–1.61 (2 H, m), 0.94 (3 H, t, J = 7.33); 13C-NMR: 199.26, 148.54, 134.63, 128.72, 128.15, 38.02, 37.32, 33.38, 32.27, 24.23, 22.88, 13.77.
5-Bromo-1-(4-iso-propylphenyl)pentan-1-one (1m). Yield: 33 %; 1H-NMR: 7.88 (2 H, d, J = 8.20), 7.30 (2 H, d, J = 8.20), 3.45 (2 H, t, J = 6.62), 2.99–2.93 (3 H, m), 1.98–1.85 (4 H, m), 1.26 (6 H, d, J = 6.94); 13C-NMR: 199.21, 154.57, 134.74, 128.28, 126.70, 37.31, 34.23, 33.38, 32.25, 23.67, 22.87.
5-Bromo-1-(4-n-butylphenyl)pentan-1-one (1n). Yield: 37 %; 1H-NMR: 7.78 (2 H, d, J = 8.33), 7.16 (2 H, d, J = 8.43), 3.36–3.33 (2 H, m), 2.90–2.87 (2 H, m), 2.59–2.55 (2 H, m), 1.89–1.75 (4 H, m), 1.56–1.48 (2 H, m), 1.31–1.22 (2 H, m), 0.84 (3 H, t, J = 7.37); 13C-NMR: 199.18, 148.77, 134.60, 128.67, 128.18, 37.31, 35.69, 33.38, 33.26, 32.29, 22.89, 22.35, 13.93.
5-Bromo-1-(4-iso-butylphenyl)pentan-1-one (1o). Yield: 51 %; 1H-NMR: 7.87 (2 H, d, J = 8.21), 7.23 (2 H, d, J = 8.14), 3.46–3.43 (2 H, m), 3.00–2.97 (2 H, m), 2.53 (2 H, d, J = 7.22), 1.97–1.86 (5 H, m), 0.90 (6 H, d, J = 6.65); 13C-NMR: 199.29, 147.61, 134.66, 129.37, 128.03, 45.41, 37.34, 33.42, 32.30, 30.15, 22.89, 22.38.
5-Bromo-1-(4-tert-butylphenyl)pentan-1-one (1p). Yield: 58 %; 1H-NMR: 7.81 (2 H, d, J = 8.66), 7.39 (2 H, d, J = 8.67), 3.37–3.34 (2 H, m), 2.91–2.88 (2 H, m), 1.90–1.77 (4 H, m), 1.25 (9 H, s); 13C-NMR: 199.22, 156.81, 134.31, 128.02, 125.58, 37.32, 35.12, 33.38, 32.27, 31.11, 22.89.
4-Bromo-1-phenylbutan-1-one (1q). Yield: 38 %; 1H-NMR: 7.85–7.83 (2 H, m), 7.45–7.41 (1 H, m), 7.34–7.31 (2 H, m), 3.41 (2 H, t, J = 6.38), 3.03 (2 H, t, J = 6.93), 2.19–2.14 (2 H, m) [Lit. [12] CDCl3: 8.00–7.96 (2 H, m), 7.60–7.50 (1 H, m), 7.48–7.44 (2 H, m), 3.55 (2 H, t, J = 6.60), 3.19 (2 H, t, J = 6.60), 2.36–2.27 (2 H, m)]; 13C-NMR: 198.36, 136.43, 132.93, 128.37, 127.72, 36.30, 33.43, 26.64.
4-Bromo-1-(4-methylphenyl)butan-1-one (1r). Yield: 52 %; 1H-NMR: 7.85 (2 H, d, J = 8.21), 7.23 (2 H, d, J = 7.92), 3.51 (2 H, t, J = 6.43), 3.11 (2 H, t, J = 6.98), 2.38 (3 H, s), 2.29–2.24 (2 H, m); 13C-NMR: 198.14, 143.80, 134.09, 129.13, 127.93, 36.23, 33.54, 26.82, 21.48.
4-Bromo-1-(4-ethylphenyl)butan-1-one (1s). Yield: 56 %; 1H-NMR: 7.90 (2 H, d, J = 8.24), 7.28 (2 H, d, J = 8.21), 3.54 (2 H, t, J = 6.44), 3.15 (2 H, t, J = 6.98), 2.73–2.68 (2 H, m), 2.32–2.27 (2 H, m), 1.26 (3 H, t, J = 7.68); 13C-NMR: 198.32, 150.15, 134.51, 128.25, 128.14, 36.47, 33.74, 28.97, 27.04, 15.25.
4-Bromo-1-(4-n-propylphenyl)butan-1-one (1t). Yield: 56 %; 1H-NMR: 7.91 (2 H, d, J = 8.26), 7.28 (2 H, d, J = 8.31), 3.55 (2 H, t, J = 6.39), 3.16 (2 H, t, J = 6.95), 2.67–2.64 (2 H, m), 2.33–2.28 (2 H, m), 1.71–1.64 (2 H, m), 0.96 (3 H, t, J = 7.40); 13C-NMR: 198.22, 148.50, 134.37, 128.58, 127.99, 37.87, 36.30, 33.57, 26.86, 24.08, 13.63.
4-Bromo-1-(4-iso-propylphenyl)butan-1-one (1u). Yield: 70 %; 1H-NMR: 7.93 (2 H, d, J = 8.34), 7.33 (2 H, d, J = 8.26), 3.55 (2 H, t, J = 6.38), 3.17 (2 H, t, J = 6.93), 3.01–2.95 (1 H, m), 2.34–2.29 (2 H, m), 1.28 (6 H, d, J = 6.93); 13C-NMR: 198.39, 154.74, 134.68, 128.29, 126.73, 36.46, 34.27, 33.68, 27.03, 23.68.
4-Bromo-1-(4-n-butylphenyl)butan-1-one (1v). Yield: 38 %; 1H-NMR: 7.80 (2 H, d, J = 8.30), 7.17 (2 H, d, J = 8.36), 3.45 (2 H, t, J = 6.40), 3.05 (2 H, t, J = 6.94), 2.58–2.55 (2 H, m), 2.22–2.17 (2 H, m), 1.55–1.49 (2 H, m), 1.30–1.22 (2 H, m), 0.84 (3 H, t, J = 7.38); 13C-NMR: 198.34, 148.90, 134.52, 128.68, 128.16, 36.46, 35.69, 33.67, 33.23, 27.04, 22.33, 13.92.
4-Bromo-1-(4-iso-butylphenyl)butan-1-one (1w). Yield: 51 %; 1H-NMR: 7.88 (2 H, d, J = 8.25), 7.22 (2 H, d, J = 8.21), 3.53 (2 H, t, J = 6.39), 3.14 (2 H, t, J = 6.94), 2.52 (2 H, d, J = 7.20), 2.31–2.26 (2 H, m), 1.93–1.85 (1 H, m), 0.90 (6 H, d, J = 6.63); 13C-NMR: 198.32, 147.60, 134.45, 129.25, 127.90, 45.28, 36.35, 33.56, 29.99, 26.92, 22.23.
4-Bromo-1-(4-tert-butylphenyl)butan-1-one (1x). Yield: 47 %; 1H-NMR: 7.92 (2 H, d, J = 8.66), 7.49 (2 H, d, J = 8.66), 3.56–3.54 (2 H, m), 3.16 (2 H, t, J = 6.93), 2.34–2.28 (2 H, m), 1.35 (9 H, s); 13C-NMR: 198.46, 156.98, 134.21, 127.99, 125.58, 36.44, 35.12, 33.66, 31.08, 27.00.
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-phenylhexan-1-one (2a). Yield: 22 %; mp 130 °C; IR 2942, 2363, 1680, 1596, 1448, 1318, 1180; 1H-NMR: 8.45 (1 H, s), 7.96–7.94 (2 H, m), 7.59–7.45 (7 H, m), 7.31–7.27 (4 H, m), 7.20–7.17 (2 H, m), 3.46 (2 H, d, J = 10.83), 3.00–2.97 (2 H, m), 2.85–2.83 (2 H, m), 2.62–2.59 (3 H, m), 2.17–2.14 (2 H, m), 1.79–1.76 (4 H, m), 1.61 (2 H, d, J = 13.78), 1.44–1.41 (2 H, m); 13C-NMR: 199.92, 167.85, 145.55, 136.88, 133.09, 128.63, 128.32, 128.01, 126.69, 125.58, 78.85, 52.58, 42.61, 37.98, 26.57, 23.93, 23.43; LC/MS-MS 442.11 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-methylphenyl)hexan-1-one (2b). Yield: 26 %; mp: 169 °C; IR: 2934, 2364, 1684, 1604, 1447, 1383, 1344, 1320, 1179; 1H-NMR: 8.44 (1 H, s), 7.81 (2 H, d, J = 8.14), 7.47 (4 H, d, J = 7.70), 7.28–7.21 (6 H, m), 7.16–7.13 (2 H, m), 3.38 (2 H, d, J = 11.19), 2.92 (2 H, t, J = 7.09), 2.78–2.75 (2 H, m), 2.60–2.49 (3 H, m), 2.38 (3 H, s), 2.14–2.06 (2 H, m), 1.75–1.69 (4 H, m), 1.56 (2 H, d, J = 13.83), 1.41–1.34 (2 H, m); 13C-NMR: 199.64, 168.13, 145.62, 143.90, 134.42, 129.32, 128.32, 128.17, 126.69, 125.62, 78.90, 52.72, 42.70, 37.92, 26.68, 24.11, 23.60, 21.67; LC/MS-MS: 456.13 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-ethylphenyl)hexan-1-one (2c). Yield: 36 %; mp: 174 °C; IR: 2939, 1685, 1605, 1447, 1383, 1345, 1320, 1286, 1148; 1H-NMR: 8.48 (1 H, s), 7.86 (2 H, d, J = 8.33), 7.51–7.49 (4 H, m), 7.30–7.26 (6 H, m), 7.19–7.16 (2 H, m), 3.38 (2 H, d, J = 11.17), 2.94 (2 H, t, J = 7.10), 2.77–2.68 (4 H, m), 2.63–2.45 (3 H, m), 2.15–2.05 (2 H, m), 1.78–1.69 (4 H, m), 1.58 (2 H, d, J = 13.44), 1.43–1.36 (2 H, m), 1.25 (3 H, t, J = 7.60); 13C-NMR: 199.65, 150.05, 145.62, 134.62, 128.31, 128.26, 128.12, 126.67, 125.59, 78.92, 42.73, 37.94, 28.95, 26.72, 24.23, 23.62, 15.23; LC/MS-MS: 470.18 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-propylphenyl)hexan-1-one (2d). Yield: 43 %; mp: 172 °C; IR: 2940, 2395, 1686, 1605, 1447, 1384, 1345, 1318, 1286, 1177; 1H-NMR: 8.27 (1 H, s), 7.64 (2 H, d, J = 8.33), 7.30–7.28 (4 H, m), 7.09–7.03 (6 H, m), 6.96 (2 H, t, J = 7.34), 3.17 (2 H, d, J = 11.10), 2.73 (2 H, t, J = 7.11), 2.56–2.53 (2 H, m), 2.44–2.23 (5 H, m), 1.95–1.85 (2 H, m), 1.57–1.35 (8 H, m), 1.22–1.16 (2 H, m), 0.73 (3 H, t, J = 7.37); 13C-NMR: 199.64, 167.99, 148.52, 145.58, 128.69, 128.28, 128.12, 126.65, 125.55, 119.97, 52.75, 43.18, 42.71, 38.00, 37.91, 26.70, 24.21, 23.58, 13.75; LC/MS-MS: 484.24 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-propylphenyl)hexan-1-one (2e). Yield: 43 %; mp: 154 °C; IR: 2936, 2360, 1686, 1606, 1446, 1382, 1345, 1317, 1286, 1181; 1H-NMR (d4-MeOH): 8.53 (1 H, s), 7.91 (2 H, d, J = 8.35), 7.53–7.51 (4 H, m), 7.36 (2 H, d, J = 8.25), 7.31–7.28 (4 H, m), 7.19–7.16 (2 H, m), 3.51 (2 H, d, J = 12.19), 3.05–2.92 (7 H, m), 2.86–2.80 (1 H, m), 1.83–1.69 (8 H, m), 1.46–1.40 (2 H, m), 1.26 (6 H, d, J = 6.93); 13C-NMR (d4-MeOH): 202.03, 156.18, 147.20, 136.14, 129.54, 129.47, 129.25, 129.19, 127.87, 127.80, 127.67, 127.13, 79.87, 53.91, 42.96, 38.90, 35.51, 27.33, 25.50, 25.16, 24.80, 24.09; LC/MS-MS: 484.24 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-butylphenyl)hexan-1-one (2f). Yield: 41 %; mp: 142 °C; IR: 2947, 2337, 1684, 1606, 1446, 1383, 1345, 1316, 1285, 1177; 1H-NMR (d4-MeOH): 8.53 (1 H, s), 7.90 (2 H, d, J = 8.27), 7.53–7.51 (4 H, m), 7.32–7.28 (6 H, m), 7.18 (2 H, t, J = 7.32), 3.52 (2 H, d, J = 12.35), 3.05–2.93 (6 H, m), 2.85–2.81 (1 H, m), 2.69–2.66 (2 H, m), 1.84–1.70 (8 H, m), 1.65–1.59 (2 H, m), 1.46–1.33 (4 H, m), 0.94 (3 H, t, J = 7.34); 13C-NMR (d4-MeOH): 204.60, 152.84, 149.71, 138.49, 132.38, 132.32, 131.92, 131.87, 131.70, 130.18, 129.63, 82.37, 56.42, 45.46, 39.13, 37.09, 29.81, 28.02, 27.66, 27.28, 25.88, 16.78; LC/MS-MS: 498.24 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-butylphenyl)hexan-1-one (2g). Yield: 43 %; mp: 201 °C; IR: 2940, 2357, 1684, 1605, 1446, 1385, 1345, 1286, 1177; 1H-NMR (d4-MeOH): 8.54 (1 H, s), 7.91 (2 H, d, J = 8.29), 7.54–7.52 (4 H, m), 7.32–7.29 (6 H, m), 7.20–7.17 (2 H, m), 3.52 (2 H, d, J = 12.22), 3.06–2.94 (6 H, m), 2.86–2.81 (1 H, m), 2.56 (2 H, d, J = 7.22), 1.96–1.72 (10 H, m), 1.48–1.42 (2 H, m), 0.92 (6 H, d, J = 6.63); 13C-NMR (d4-MeOH): 200.20, 149.13, 147.16, 136.06, 130.54, 129.18, 127.68, 127.12, 79.88, 53.96, 46.29, 42.97, 31.35, 27.27, 25.55, 25.52, 25.18, 24.71, 22.67; LC/MS-MS: 498.23 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-tert-butylphenyl)hexan-1-one (2h). Yield 56 %; mp 67 °C; IR 2962, 2359, 1677, 1603, 1447, 1271, 1191; 1H-NMR (d4-MeOH) 8.48 (1 H, s), 7.92 (2 H, d, J = 8.49), 7.54–7.51 (6 H, m), 7.31–7.28 (4 H, m), 7.18 (2 H, t, J = 7.30), 3.53 (2 H, d, J = 12.10), 3.07–2.96 (6 H, m), 2.86–2.81 (1 H, m), 1.84–1.74 (8 H, m), 1.47–1.41 (2 H, m), 1.34 (9 H, s); 13C-NMR (d4-MeOH) 202.08, 158.30, 147.16, 135.67, 129.19, 127.65, 127.11, 126.73, 112.07, 79.86, 53.87, 42.89, 38.89, 36.00, 31.49, 27.26, 25.07, 24.80, 24.77; LC/MS-MS 498.23 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-phenylpentan-1-one (2i). Yield: 25 %; mp: 143 °C; IR: 3059, 2954, 2361, 1682, 1595, 1446, 1388, 1341, 1206; 1H-NMR: 8.31 (1 H, s), 7.81–7.79 (2 H, m), 7.45–7.42 (1 H, m), 7.38–7.37 (4 H, m), 7.35–7.32 (2 H, m), 7.17–7.14 (4 H, m), 7.06–7.03 (2 H, m), 3.33 (2 H, d, J = 11.67), 2.92–2.90 (2 H, m), 2.76–2.73 (2 H, m), 2.51–2.43 (3 H, m), 2.05–1.96 (2 H, m), 1.70–1.61 (4 H, m), 1.47 (2 H, d, J = 14.19); 13C-NMR: 199.46, 145.57, 136.68, 133.22, 128.66, 128.30, 127.98, 126.67, 125.58, 78.84, 52.64, 42.63, 37.60, 23.76, 21.24; LC/MS-MS: 428.10 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-methylphenyl)pentan-1-one (2j). Yield: 40 %; mp: 165 °C; IR: 2953, 2358, 1679, 1592, 1445, 1387, 1343, 1318, 1180; 1H-NMR (CDCl3 + d4-MeOH): 8.44 (1 H, s), 7.76 (2 H, d, J = 8.28), 7.43 (4 H, d, J = 7.28), 7.23–7.17 (6 H, m), 7.11–7.07 (2 H, m), 3.42 (2 H, d, J = 12.05), 2.97–2.85 (4 H, m), 2.58–2.52 (3 H, m), 2.33 (3 H, s), 2.03–1.90 (2 H, m), 1.72–1.67 (4 H, m), 1.52 (2 H, d, J = 14.56); 13C-NMR (CDCl3 + d4-MeOH): 199.21, 168.45, 145.32, 143.90, 133.78, 129.02, 127.93, 127.79, 126.25, 125.16, 78.01, 52.32, 42.18, 37.02, 23.13, 23.11, 23.06, 21.26, 20.79, 20.74; LC/MS-MS: 442.20 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-ethylphenyl)pentan-1-one (2k). Yield: 30 %; mp: 167 °C; IR 2953, 2362, 1678, 1605, 1446, 1388, 1342, 1318, 1178; 1H-NMR (CDCl3 + d4-MeOH): 8.38 (1 H, s), 7.78 (2 H, d, J = 8.34), 7.42 (4 H, d, J = 9.52), 7.23–7.19 (6 H, m), 7.11–7.08 (2 H, m), 3.36 (2 H, d, J = 11.60), 2.94–2.91 (2 H, m), 2.79–2.75 (2 H, m), 2.63 (2 H, q, J = 7.60), 2.56–2.40 (3 H, m), 2.03 (2 H, d, J = 12.51), 1.72–1.69 (4 H, m), 1.52 (2 H, d, J = 13.92), 1.18 (3 H, t, J = 7.62); 13C-NMR (CDCl3 + d4-MeOH): 199.16, 168.05, 150.19, 145.59, 134.44, 128.29, 128.21, 128.14, 126.65, 125.58, 78.86, 52.66, 42.67, 37.52, 30.92, 28.92, 23.87, 23.53, 21.38, 15.17; LC/MS-MS: 465.20 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-propylphenyl)pentan-1-one (2l). Yield: 37 %; mp: 166 °C; IR: 3059, 2954, 2357, 1679, 1605, 1446, 1384, 1341, 1315, 1179 1H-NMR: 8.37 (1 H, s), 7.77 (2 H, d, J = 8.24), 7.42 (4 H, d, J = 7.91), 7.22–7.16 (6 H, m), 7.10–7.07 (2 H, m), 3.34 (2 H, d, J = 11.46), 2.93–2.90 (2 H, m), 2.75–2.73 (2 H, m), 2.57–2.42 (5 H, m), 2.06–1.97 (2 H, m), 1.68–1.48 (8 H, m), 0.86 (3 H, t, J = 7.31); 13C-NMR: 199.19, 168.15, 148.67, 145.67, 134.46, 128.74, 128.26, 128.12, 126.62, 125.61, 78.84, 52.62, 42.70, 38.00, 37.54, 24.19, 23.89, 23.57, 21.40, 13.75; LC/MS-MS: 470.22 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-propylphenyl)pentan-1-one (2m). Yield: 33 %; mp: 166 °C; IR: 2963, 2360, 1679, 1596, 1447, 1389, 1342, 1317, 1188; 1H-NMR: 8.37 (1 H, s), 7.80 (2 H, d, J = 8.20), 7.42 (4 H, d, J = 8.20), 7.23–7.19 (6 H, m), 7.11–7.08 (2 H, m), 3.34 (2 H, d, J = 11.98), 2.93–2.85 (3 H, m), 2.78–2.75 (2 H, m), 2.54–2.44 (3 H, m), 2.06–1.99 (2 H, m), 1.69–1.68 (4 H, m), 1.51 (2 H, d, J = 13.87), 1.18 (6 H, d, J = 6.94); 13C-NMR: 199.17, 168.13, 154.75, 145.63, 134.58, 128.29, 128.26, 126.73, 125.59, 126.64, 78.85, 42.68, 37.53, 34.24, 23.86, 23.65, 21.38; LC/MS-MS: 470.30 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)-piperidin-1-yl)-1-(4-n-butylphenyl)pentan-1-one (2n). Yield: 28 %; mp: 157 °C; IR: 2955, 2365, 1679, 1604, 1447, 1387, 1341, 1314, 1248, 1183; 1H-NMR: 8.37 (1 H, s), 7.77 (2 H, d, J = 8.24), 7.42 (4 H, d, J = 7.41), 7.22–7.17 (6 H, m), 7.11–7.07 (2 H, m), 3.37 (2 H, d, J = 11.56), 2.94–2.91 (2 H, m), 2.79–2.76 (2 H, m), 2.60–2.45 (5 H, m), 2.09–2.00 (2 H, m), 1.71–1.65 (4 H, m), 1.57–1.49 (4 H, m), 1.32–1.24 (2 H, m), 0.85 (3 H, t, J = 7.29); 13C-NMR: 199.17, 168.09, 148.96, 145.62, 134.41, 128.70, 128.30, 128.14, 126.65, 125.59, 78.85, 52.64, 42.66, 37.52, 35.69, 33.23, 23.84, 23.51, 22.32, 21.37, 13.91; LC/MS-MS: 484.34 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)-piperidin-1-yl)-1-(4-iso-butylphenyl)pentan-1-one (2o). Yield: 29 %; mp: 173 °C; IR: 2953, 2365, 1679, 1605, 1447, 1387, 1341, 1318, 1234, 1187; 1H-NMR: 8.47 (1 H, s), 7.87–7.85 (2 H, m), 7.52–7.50 (4 H, m), 7.30–7.19 (8 H, m), 3.45–3.43 (2 H, m), 3.03–3.01 (2 H, m), 2.85–2.84 (2 H, m), 2.64–2.53 (5 H, m), 2.16–2.11 (2 H, m), 1.94–1.89 (1 H, m), 1.78 (4 H, m), 1.60 (2 H, d, J = 13.64), 0.93–0.91 (6 H, m); 13C-NMR: 199.19, 167.96, 147.75, 145.56, 134.51, 129.38, 128.32, 127.99, 126.69, 125.58, 78.90, 52.69, 45.39, 42.71, 37.53, 30.10, 23.91, 23.57, 22.34, 21.41; LC/MS-MS: 484.34 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)-piperidin-1-yl)-1-(4-tert-butylphenyl)pentan-1-one (2p). Yield: 26 %; mp: 171 °C; IR: 2964, 2365, 1684, 1588, 1446, 1380, 1342, 1322, 1230, 1188; 1H-NMR: 8.38 (1 H, s), 7.81–7.79 (2 H, m), 7.43–7.39 (6 H, m), 7.23–7.20 (4 H, m), 7.12–7.09 (2 H, m), 3.37–3.35 (2 H, d, J = 10.49), 2.94–2.92 (2 H, m), 2.77–2.76 (2 H, m), 2.55–2.45 (3 H, m), 2.08–2.01 (1 H, m), 1.70 (4 H, m), 1.52 (2 H, d, J = 14.12), 1.26 (9 H, s); 13C-NMR: 199.16, 167.91, 156.99, 145.55, 134.16, 128.32, 127.97, 126.70, 125.60, 125.58, 78.90, 52.73, 52.69, 42.70, 37.54, 35.13, 31.09, 23.89, 23.58, 23.55, 21.42; LC/MS-MS: 484.31 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-phenylbutan-1-one (2q). Yield: 18 %; mp: 175 °C; IR: 2358, 1681, 1596, 1489, 1447, 1365, 1345, 1065; 1H-NMR (d4-MeOH): 8.53 (1 H, s), 8.00–7.99 (2 H, m), 7.61 (1 H, t, J = 7.41), 7.54–7.49 (6 H, m), 7.32–7.28 (4 H, m), 7.18 (2 H, t, J = 7.36), 3.57 (2 H, d, J = 12.26), 3.20–3.17 (2 H, m), 3.12–3.09 (2 H, m), 3.01–2.96 (2 H, m), 2.88–2.82 (1 H, m), 2.13–2.07 (2 H, m), 1.86–1.72 (4 H, m) [Lit. [14] 200 MHz, CDCl3: 7.90–7.05 (15 H, m), 2.89 (4 H, m), 2.31 (3 H, m), 1.98–1.77 (4 H, m), 1.52–1.20 (4 H, m)]; 13C-NMR (d4-MeOH): 198.98, 145.82, 136.52, 133.16, 128.42, 127.79, 127.71, 126.26, 125.71, 78.49, 55.98, 52.63, 47.64, 41.59, 34.77, 24.17, 18.35; LC/MS-MS: 414.18 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-methylphenyl)butan-1-one (2r). Yield: 13 %; mp: 191 °C; IR: 2358, 1681, 1596, 1489, 1447, 1365, 1345, 1065; 1H-NMR (d4-MeOH): 8.53 (1 H, s), 7.89 (2 H, d, J = 8.24), 7.54–7.52 (4 H, m), 7.32–7.28 (6 H, m), 7.18 (2 H, t, J = 7.35), 3.56 (2 H, d, J = 12.32), 3.16–3.08 (4 H, m), 3.00–2.95 (2 H, m), 2.88–2.82 (1 H, m), 2.40 (3 H, s), 2.10–2.06 (2 H, m), 1.86–1.71 (4 H, m); 13C-NMR (d4-MeOH): 200.22, 147.24, 145.74, 133.45, 130.44, 129.30, 129.21, 127.67, 127.12, 79.90, 54.03, 43.00, 25.57, 21.66, 19.76, 19.70; LC/MS-MS: 428.32 (M+H+) [Lit. [15] 428 (M+H+)].
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-ethylphenyl)butan-1-one (2s). Yield: 20 %; mp: 204 °C; IR: 2968, 2358, 1687, 1606, 1446, 1388, 1346, 1183; 1H-NMR (d4-MeOH): 8.52 (1 H, s), 7.92 (2 H, d, J = 8.33), 7.54–7.52 (4 H, m), 7.35–7.28 (6 H, m), 7.19–7.16 (2 H, m), 3.56 (2 H, d, J = 12.34), 3.17–3.08 (4 H, m), 3.01–2.95 (2 H, m), 2.87–2.81 (1 H, m), 2.74–2.69 (2 H, m), 2.12–2.06 (2 H, m), 1.85–1.72 (4 H, m), 1.25 (3 H, t, J = 7.62); 13C-NMR (d4-MeOH): 200.19, 151.95, 147.19, 135.67, 129.40, 129.28, 129.18, 127.67, 127.12, 79.91, 54.09, 43.02, 36.08, 29.89, 25.62, 19.87, 15.70; LC/MS-MS: 442.32 (M+H+) [Lit. [15] 442 (M+H+)].
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-propylphenyl)butan-1-one (2t). Yield: 10 %; mp: 182 °C; IR: 3182, 2359, 1684, 1602, 1446, 1388, 1347; 1H-NMR (d4-MeOH): 8.54 (1 H, s), 7.92 (2 H, d, J = 8.31), 7.55–7.53 (4 H, m), 7.34–7.29 (6 H, m), 7.20–7.17 (2 H, t, J = 7.35), 3.56 (2 H, d, J = 12.20), 3.18–3.08 (4 H, m), 3.00–2.95 (2 H, m), 2.87–2.82 (1 H, m), 2.69–2.66 (2 H, m), 2.11–2.07 (2 H, m), 1.83–1.64 (6 H, m), 0.95 (3 H, t, J = 7.38); 13C-NMR (d4-MeOH): 200.21, 150.33, 147.21, 135.71, 129.91, 129.31, 129.18, 127.66, 127.12, 79.91, 57.50, 54,08, 43.04, 38.96, 36.10, 25.62, 25.37, 19.89, 19.83, 14.02; LC/MS-MS: 456.24 (M+H+) [Lit. [15] 456 (M+H+)].
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-propylphenyl)butan-1-one (2u). Yield: 32 %; mp: 189 °C; IR: 2960, 2366, 1691, 1596, 1446, 1345, 1189, 1062; 1H-NMR (d4-MeOH): 8.52 (1 H, s), 7.92 (2 H, d, J = 8.38), 7.54–7.52 (4 H, m), 7.37 (2 H, d, J = 8.26), 7.30 (4 H, t, J = 7.76), 7.19–7.16 (2 H, m), 3.56 (2 H, d, J = 12.30), 3.17–3.08 (4 H, m), 3.00–2.95 (3 H, m), 2.87–2.81 (1 H, m), 2.11–2.06 (2 H, m), 1.85–1.72 (4 H, m), 1.27 (6 H, t, J = 6.92); 13C-NMR (d4-MeOH): 200.24, 156.44, 147.21, 135.82, 129.45, 129.19, 127.86, 127.67, 127.12, 79.90, 57.43, 54,06, 43.02, 36.10, 35.53, 25.59, 24.04, 19.80; LC/MS-MS: 456.38 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-butylphenyl)butan-1-one (2v). Yield: 6 %; mp: 161 °C; IR: 2963, 1687, 1607, 1448, 1362, 1182, 1139; 1H-NMR (d4-MeOH): 8.54 (1 H, s), 7.91 (2 H, d, J = 8.30), 7.54–7.52 (4 H, m), 7.33–7.28 (6 H, m), 7.18 (2 H, t, J = 7.33), 7.19–7.16 (2 H, m), 3.55 (2 H, d, J = 12.29), 3.16–3.06 (4 H, m), 2.98–2.93 (2 H, m), 2.87–2.81 (1 H, m), 2.70–2.67 (2 H, m), 2.12–2.06 (2 H, m), 1.85–1.71 (4 H, m), 1.65–1.59 (2 H, m), 1.40–1.32 (2 H, m), 0.94 (3 H, t, J = 7.40); 13C-NMR (d4-MeOH): 200.23, 150.56, 147.23, 135.67, 129.85, 129.32, 129.18, 127.65, 127.12, 79.92, 57.50, 54,08, 43.07, 36.61, 36.11, 34.52, 25.62, 23.33, 19.91, 14.22; LC/MS-MS: 470.17 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-butylphenyl)butan-1-one (2w). Yield: 20 %; mp: 155 °C; IR: 2956, 1689, 1605, 1448, 1385, 1316, 1193; 1H-NMR (d4-MeOH): 8.55 (1 H, s), 7.93 (2 H, d, J = 8.28), 7.55–7.53 (4 H, m), 7.33–7.29 (6 H, m), 7.19 (2 H, t, J = 7.33), 3.56 (2 H, d, J = 12.35), 3.18–3.08 (4 H, m), 3.00–2.95 (2 H, m), 2.88–2.83 (1 H, m), 2.56 (2 H, d, J = 7.23), 2.13–2.07 (2 H, m), 1.96–1.73 (5 H, m), 0.92 (6 H, d, J = 6.63); 13C-NMR (d4-MeOH): 195.87, 149.37, 147.20, 135.75, 130.54, 129.18, 127.66, 127.12, 79.92, 54.11, 46.30, 43.05, 36.10, 31.35, 25.66, 22.66, 19.90, 19.85; LC/MS-MS: 470.17 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-tert-butylphenyl)butan-1-one (2x). Yield: 27 %; mp: 175 °C; IR: 2961, 2330, 1691, 1590, 1447, 1383, 1346, 1192; 1H-NMR (d4-MeOH): 8.39 (1 H, s), 7.93 (2 H, d, J = 8.58), 7.55–7.53 (6 H, m), 7.31–7.28 (4 H, m), 7.19–7.16 (2 H, m), 3.59 (2 H, d, J = 12.22), 3.18–3.12 (4 H, m), 3.04–3.00 (2 H, m), 2.89–2.84 (1 H, m), 2.13–2.07 (2 H, m), 1.89–1.80 (2 H, m), 1.74–1.71 (2 H, m), 1.34 (9 H, s); 13C-NMR (d4-MeOH): 200.12, 168.28, 158.52, 147.21, 135.34, 129.21, 127.67, 127.10, 126.75, 79.87, 53.88, 42.88, 36.08, 36.02, 31.49, 25.40, 19.66; LC/MS-MS: 470.03 (M+H+) [Lit. 470 (M+H+)] [15].
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-phenylhexan-1-ol (3a). Yield: 83 %; mp: 80 °C; IR: 2941, 2503, 1492, 1448, 1345, 1066; 1H-NMR (d4-MeOH): 7.52 (4 H, d, J = 7.80), 7.32–7.28 (8 H, m), 7.24–7.21 (1 H, m), 7.19–7.16 (2 H, m), 4.61–4.59 (1 H, m), 3.52 (2 H, d, J = 12.23), 3.03–2.96 (4 H, m), 2.88–2.82 (1 H, m), 1.82–1.66 (8 H, m), 1.49–1.29 (4 H, m); 13C-NMR (d4-MeOH): 146.97, 146.31, 129.16, 129.05, 128.12, 127.53, 126.95, 126.91, 79.65, 74.79, 58.06, 53.90, 42.67, 39.71, 27.44, 26.21, 25.61, 24.93; LC/MS-MS: 444.21 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-methylphenyl)hexan-1-ol (3b). Yield: 62 %; mp: 71 °C; IR: 2941, 2361, 1593, 1491, 1447, 1335, 1066; 1H-NMR (d4-MeOH): 8.53 (1 H, s), 7.51 (4 H, d, J = 7.39), 7.31–7.28 (4 H, m), 7.21–7.16 (4 H, m), 7.12 (2 H, d, J = 7.92), 4.55 (1 H, t, J = 6.62), 3.48 (2 H, d, J = 12.38), 2.98–2.89 (4 H, m), 2.84–2.78 (1 H, m), 2.30 (3 H, s), 1.81–1.63 (8 H, m), 1.47–1.27 (4 H, m); 13C-NMR (d4-MeOH): 170.17, 147.20, 143.39, 137.98, 129.91, 129.20, 127.68, 127.12, 127.07, 79.88, 74.85, 53.92, 42.99, 39.82, 27.68, 26.42, 25.56, 25.53, 25.24, 21.17; LC/MS-MS: 458.21 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-ethylphenyl)hexan-1-ol (3c). Yield: 82 %; mp: 75 °C; IR: 2936, 2364, 1595, 1447, 1359, 1066; 1H-NMR (d4-MeOH): 8.53 (1 H, s), 7.53–7.51 (4 H, m), 7.31–7.27 (4 H, m), 7.22 (2 H, d, J = 8.04), 7.19–7.14 (4 H, m), 4.56 (1 H, t, J = 6.62), 3.48 (2 H, d, J = 12.67), 2.99–2.90 (4 H, m), 2.85–2.79 (1 H, m), 2.63–2.59 (2 H, q, J = 7.61), 1.83–1.63 (8 H, m), 1.47–1.27 (4 H, m), 1.21 (3 H, t, J = 7.59); 13C-NMR (d4-MeOH): 147.21, 144.55, 143.66, 129.20, 128.76, 127.67, 127.15, 127.11, 79.87, 74.87, 53.87, 42.95, 39.82, 29.57, 27.67, 26.43, 25.50, 25.18, 16.32; LC/MS-MS: 472.21 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-propylphenyl)hexan-1-ol (3d). Yield: 71 %; mp: 73 °C; IR: 2933, 2364, 1596, 1448, 1334, 1179, 1064; 1H-NMR (d4-MeOH): 8.54 (1 H, s), 7.52–7.51 (4 H, m), 7.31–7.27 (4 H, m), 7.22 (2 H, d, J = 8.05), 7.19–7.12 (4 H, m), 4.56 (1 H, t, J = 6.55), 3.45 (2 H, d, J = 12.32), 2.95–2.78 (5 H, m), 2.57–2.54 (2 H, m), 1.80–1.58 (10 H, m), 1.46–1.28 (4 H, m), 0.92 (3 H, t, J = 7.38); 13C-NMR (d4-MeOH): 145.80, 142.28, 141.43, 127.98, 127.76, 126.23, 125.69, 125.63, 120.00, 79.47, 73.45, 56.54, 52.53, 41.65, 38.39, 37.32, 26.28, 25.03, 24.41, 24.17, 23.88, 12.68; LC/MS-MS: 486.27 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-propylphenyl)hexan-1-ol (3e). Yield: 75 %; mp: 62 °C; IR: 2961, 2364, 1597, 1448, 1333, 1180, 1064; 1H-NMR (d4-MeOH): 8.53 (1 H, s), 7.51 (4 H, d, J = 8.15), 7.31–7.28 (4 H, m), 7.23 (2 H, d, J = 8.12), 7.19–7.16 (4 H, m), 4.58–4.55 (1 H, m), 3.47 (2 H, d, J = 11.87), 2.98–2.79 (6 H, m), 1.80–1.63 (7 H, m), 1.47–1.24 (4 H, m), 1.23 (6 H, d, J = 6.93); 13C-NMR (d4-MeOH): 149.17, 147.19, 143.81, 129.20, 127.68, 127.29, 127.12, 79.88, 74.86, 53.91, 42.98, 39.81, 35.14, 27.68, 26.45, 25.54, 25.23, 24.54; LC/MS-MS: 486.34 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-butylphenyl)hexan-1-ol (3f). Yield: 55 %; mp: 64 °C; IR 2930, 2356, 1595, 1448, 1341, 1068; 1H-NMR (d4-MeOH): 8.54 (1 H, s), 7.51 (4 H, d, J = 7.37), 7.31–7.27 (4 H, m), 7.22 (2 H, d, J = 8.04), 7.19–7.12 (4 H, m), 4.56 (1 H, m), 3.44 (2 H, d, J = 12.00), 2.93–2.76 (5 H, m), 2.58 (2 H, t, J = 7.72), 1.79–1.54 (10 H, m), 1.46–1.29 (6 H, m), 0.93 (3 H, t, J = 7.40); 13C-NMR (d4-MeOH): 147.24, 143.66, 143.08, 129.35, 129.19, 127.67, 127.14, 79.91, 74.90, 54.01, 43.14, 39.81, 36.33, 35.07, 27.74, 26.47, 25.67, 25.38, 23.36, 14.32; LC/MS-MS: 500.45 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-butylphenyl)hexan-1-ol (3g). Yield: 76 %; mp: 70 °C; IR: 2953, 1596, 1447, 1339, 1168, 1065; 1H-NMR (d4-MeOH): 8.55 (1 H, s), 7.53–7,52 (4 H, m), 7.32–7.29 (4 H, m), 7.25–7.17 (4 H, m), 7.11 (2 H, d, J = 8.05), 4.59–4.57 (1 H, m), 3.48 (2 H, d, J = 12.41), 2.98–2.79 (5 H, m), 2.47–2.45 (2 H, d, J = 7.18), 1.87–1.64 (9 H, m), 1.49–1.29 (4 H, m), 0.90 (6 H, d, J = 6.62); 13C-NMR (d4-MeOH): 147.17, 143.76, 141.87, 130.05, 129.18, 127.67, 127.11, 126.93, 79.89, 74.88, 53.94, 46.13, 43.01, 39.76, 31.50, 27.66, 26.43, 25.25, 22.71, 19.23; LC/MS-MS: 500.19 (M+H+).
6-(4'-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-tert-butylphenyl)hexan-1-ol (3h). Yield: 69 %; mp: 73 °C; IR: 2957, 2365, 1597, 1447, 1341, 1171, 1066; 1H-NMR (d4-MeOH): 8.52 (1 H, s), 7.53–7.52 (4 H, m), 7.37 (2 H, d, J = 8.41), 7.32–7.25 (6 H, m), 7.20–7.17 (2 H, m), 4.59–4.57 (1 H, m), 3.50 (2 H, d, J = 12.34), 3.02–2.94 (4 H, m), 2.86–2.80 (1 H, m), 1.83–1.65 (8 H, m), 1.49–1.32 (4 H, m), 1.31 (9 H, s); 13C-NMR (d4-MeOH): 169.85, 151.33, 147.13, 143.35, 129.20, 127.69, 127.10, 126.84, 126.16, 79.88, 74.76, 53.87, 49.04, 42.90, 39.74, 35.32, 31.85, 27.63, 26.41, 25.14; LC/MS-MS: 500.33 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-phenylpentan-1-ol (3i). Yield: 75 %; mp: 182 °C; IR: 2778, 2361, 1629, 1448, 1356, 1064; 1H-NMR (d4-MeOH): 8.55 (2 H, s), 7.52–7.50 (4 H, m), 7.34–7.27 (8 H, m), 7.24–7.20 (1 H, m), 7.18–7.15 (2 H, m), 4.61 (1 H, t, J = 5.78), 3.45 (2 H, d, J = 12.37), 2.95–2.76 (5 H, m), 1.84–1.65 (8 H, m), 1.51–1.29 (3 H, m); 13C-NMR (d4-MeOH): 147.20, 146.29, 129.34, 129.15, 128.32, 127.63, 127.11, 127.02, 79.89, 74.71, 53.93, 43.11, 39.53, 25.56, 25.28, 24.13; LC/MS-MS: 430.14 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-methylphenyl)pentan-1-ol (3j). Yield: 71 %; mp: 84 °C; IR: 3340, 2951, 2361, 1595, 1491, 1447, 1330, 1173; 1H-NMR: 8.41 (1 H, s), 7.49 (4 H, d, J = 7.53), 7.29–7.25 (4 H, m), 7.20–7.09 (6 H, m), 4.61–4.60 (1 H, m), 3.41–3.38 (2 H, m), 2.82–2.80 (2 H, m), 2.58–2.55 (3 H, m), 2.31 (3 H, s), 2.15–2.08 (2 H, m), 1.82–1.67 (4 H, m), 1.54 (2 H, d, J = 13.11), 1.44–1.35 (2 H, m); 13C-NMR: 168.26, 145.65, 141.87, 136.90, 129.04, 128.29, 126.62, 125.78, 125.59, 78.74, 73.28, 52.47, 42.43, 38.27, 23.64, 23.05, 21.12; LC/MS-MS: 444.38 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-ethylphenyl)pentan-1-ol (3k). Yield: 80 %; mp: 70 °C; IR: 2933, 1595, 1447, 1341, 1065; 1H-NMR (d4-MeOH): 8.46 (1 H, s), 7.49 (4 H, d, J = 8.27), 7.28–7.25 (4 H, m), 7.21 (2 H, d, J = 8.01), 7.16–7.12 (4 H, m), 4.56 (1 H, t, J = 6.90), 3.46 (2 H, d, J = 12.17), 2.99–2.90 (4 H, m), 2.82–2.78 (1 H, m), 2.58 (1 H, q, J = 7.58), 1.79–1.65 (8 H, m), 1.45–1.27 (2 H, m), 1.17 (3 H, t, J = 7.59); 13C-NMR (d4-MeOH): 145.74, 143.24, 142.06, 127.79, 127.40, 126.28, 125.69, 120.02, 78.43, 73.19, 52.47, 41.45, 37.99, 36.34, 28.15, 23.67, 22.65, 14.91; LC/MS-MS: 458.16 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-propylphenyl)pentan-1-ol (3l). Yield: 64 %; mp: 83 °C; IR: 2933, 2362, 1594, 1447, 1342, 1242, 1066; 1H-NMR (d4-MeOH): 8.50 (1 H, s), 7.50–7.48 (4 H, m), 7.28–7.25 (4 H, m), 7.21–7.20 (2 H, m), 7.15–7.10 (4 H, m), 4.56–4.55 (1 H, m), 3.44 (2 H, d, J = 12.59), 2.94–2.87 (4 H, m), 2.81–2.76 (1 H, m), 2.54–2.52 (1 H, m), 1.75–1.58 (10 H, m), 1.44–1.27 (2 H, m), 0.91–0.88 (3 H, m); 13C-NMR (d4-MeOH): 147.02, 143.36, 142.79, 138.87, 129.47, 129.29, 129.03, 127.51, 127.38, 126.94, 126.86, 79.69, 74.46, 53.69, 42.78, 39.24, 38.57, 25.68, 25.32, 24.98, 23.93, 13.94; LC/MS-MS: 472.23 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-propylphenyl)pentan-1-ol (3m). Yield: 68 %; mp: 82 °C; IR: 3337, 2956, 2298, 1597, 1447, 1385, 1179; 1H-NMR: 8.40 (1 H, s), 7.49 (2 H, d, J = 7.54), 7.30–7.23 (6 H, m), 7.19–7.17 (4 H, m), 4.66–4.63 (1 H, m), 3.47–3.45 (2 H, m), 2.90–2.86 (3 H, m), 2.63–2.59 (3 H, m), 2.21–2.17 (2 H, m), 1.82–1.68 (4 H, m), 1.59 (2 H, d, J = 13.79). 1.51–1.41 (2 H, m), 1.23 (6 H, d, J = 6.92); 13C-NMR: 167.61, 148.08, 145.45, 142.05, 128.34, 126.71, 126.45, 125.82, 125.56, 78.82, 73.46, 42.43, 38.05, 33.79, 24.02, 23.55, 23.05; LC/MS-MS: 472.38 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)-piperidin-1-yl)-1-(4-n-butylphenyl)pentan-1-ol (3n). Yield: 71 %; IR: 2930, 2362, 1592, 1448, 1343, 1066; 1H-NMR (d4-MeOH): 8.34 (1 H, s), 7.51 (4 H, d, J = 8.30), 7.31–7.27 (4 H, m), 7.23 (2 H, d, J = 8.04), 7.19–7.12 (4 H, m), 4.58 (1 H, t, J = 6.20), 3.49 (2 H, d, J = 12.36), 3.02–2.79 (5 H, m), 2.58 (2 H, t, J = 7.72), 1.83–1.66 (8 H, m), 1.60–1.48 (2 H, m), 1.31–1.29 (4 H, m), 0.92 (3 H, t, J = 7.32); 13C-NMR (d4-MeOH): 166.41, 145.71, 142.05, 141.77, 127.99, 127.80, 126.29, 125.69, 125.63, 78.43, 73.21, 52.43, 41.42, 37.96, 34.89, 33.65, 23.67, 22.64, 21.94, 12.89; LC/MS-MS: 486.20 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)-piperidin-1-yl)-1-(4-iso-butylphenyl)pentan-1-ol (3o). Yield: 68 %; mp: 75 °C; IR: 2952, 2504, 1593, 1447, 1345, 1186, 1067; 1H-NMR (d4-MeOH): 8.36 (1 H, s), 7.53–7.50 (4 H, m), 7.31–7.27 (4 H, m), 7.24 (2 H, d, J = 8.02), 7.19–7.16 (2 H, m), 7.11 (2 H, d, J = 8.11), 4.59 (1 H, t, J = 5.99), 3.49 (2 H, d, J = 12.54), 3.03–2.80 (5 H, m), 2.44 (2 H, d, J = 7.18), 1.86–1.67 (9 H, m), 1.50–1.29 (2 H, m), 0.88 (6 H, d, J = 6.63); 13C-NMR (d4-MeOH): 147.12, 143.58, 141.96, 130.10, 129.20, 127.69, 127.10, 126.90, 79.84, 74.61, 53.88, 46.11, 42.82, 39.34, 31.51, 25.07, 24.06, 22.70; LC/MS-MS: 486.27 (M+H+).
5-(4’-(Hydroxydiphenylmethyl)-piperidin-1-yl)-1-(4-tert-butylphenyl)pentan-1-ol (3p). Yield: 58 %; mp: 88 °C; IR: 2950, 2359, 1597, 1447, 1345, 1033; 1H-NMR (d4-MeOH): 8.46 (1 H, s), 7.52–7.50 (4 H, m), 7.36 (2 H, d, J = 8.43), 7.31–7.25 (6 H, m), 7.19–7.16 (2 H, m), 4.60 (1 H, t, J = 6.84), 3.49 (2 H, d, J = 12.54), 3.02–2.94 (4 H, m), 2.86–2.80 (1 H, m), 1.84–1.67 (8 H, m), 1.49–1.32 (2 H, m), 1.29 (9 H, s); 13C-NMR (d4-MeOH): 153.94, 149.62, 145.67, 131.73, 130.23, 130.19, 129.60, 129.36, 129.33, 128.74, 82.38, 77.04, 56.43, 45.35, 41.83, 37.85, 34.37, 27.61, 26.54; LC/MS-MS: 486.27 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-phenylbutan-1-ol (3q). Yield: 76 %; mp: 94 °C; IR: 2778, 2361, 2339, 1595, 1448, 1356, 1064; 1H-NMR (d4-MeOH): 8.53 (1 H, s), 7.51 (4 H, d, J = 7.56), 7.36–7.23 (9 H, m), 7.17 (2 H, d, J = 7.29), 4.68–4.66 (1 H, m), 3.45–3.44 (2 H, m), 3.02–2.99 (2 H, m), 2.92–2.78 (3 H, m), 1.84–1.67 (8 H, m) [Lit. [14] 200 MHz, CDCl3: 7.67–7.11 (15 H, m), 4.60 (1 H, m), 3.43 (1 H, d, J = 7.00), 3.12 (1 H, d, J = 11.00), 2.94 (1 H, d, J = 11.00), 2.51–2.34 (5 H, m), 2.15–1.74 (4 H, m), 1.62–1.44 (4 H, m)]; 13C-NMR (d4-MeOH): 147.21, 145.98, 129.43, 129.17, 128.46, 127.65, 127.09, 126.91, 79.89, 74.27, 54.03, 53.91, 43.05, 37.21, 25.57, 22.08, 21.89; LC/MS-MS: 416.21 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-methylphenyl)butan-1-ol (3r). Yield: 45 %; mp: 97 °C; IR: 2355, 1595, 1447, 1356, 1063; 1H-NMR (d4-MeOH): 8.54 (1 H, s), 7.52 (4 H, d, J = 7.90), 7.32–7.29 (4 H, m), 7.24 (2 H, d, J = 8.00), 7.20–7.14 (4 H, m), 4.65–4.63 (1 H, m), 3.48–3.45 (2 H, m), 3.04–3.00 (2 H, m), 2.94–2.79 (3 H, m), 2.31 (3 H, s), 1.83–1.68 (8 H, m); 13C-NMR (d4-MeOH): 170.06, 147.18, 142.87, 138.23, 130.02, 129.18, 127.66, 127.09, 126.89, 79.89, 74.14, 54.03, 53.91, 43.02, 37.13, 25.60, 22.08, 21.89, 21.12; LC/MS-MS: 430.14 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-ethylphenyl)butan-1-ol (3s). Yield: 41 %; mp: 99 °C; IR: 2959, 1596, 1490, 1447, 1344, 1066; 1H-NMR (d4-MeOH): 8.55 (1 H, s), 7.52 (4 H, d, J = 7.83), 7.31–7.25 (6 H, m), 7.19–7.17 (4 H, m), 4.65–4.63 (1 H, m), 3.41–3.36 (2 H, m), 2.95–2.91 (2 H, m), 2.79–2.76 (3 H, m), 2.65–2.60 (2 H, m), 1.82–1.66 (8 H, m), 1.21 (3 H, t, J = 7.61); 13C-NMR (d4-MeOH): 170.26, 147.31, 144.78, 143.23, 143.21, 129.17, 128.88, 127.63, 127.13, 127.01, 79.97, 74.28, 74.17, 58.31, 54.21, 54.08, 43.36, 37.38, 29.56, 25.82, 22.39, 22.19, 16.29; LC/MS-MS: 444.28 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-propylphenyl)butan-1-ol (3t). Yield: 76 %; mp: 104 °C; IR: 2933, 2363, 1593, 1491, 1447, 1342, 1065; 1H-NMR (d4-MeOH): 8.56 (1 H, s), 7.52 (4 H, d, J = 7.74), 7.31–7.25 (6 H, m), 7.19–7.15 (4 H, m), 4.64–4.63 (1 H, m), 3.38–3.32 (2 H, m), 2.90–2.86 (2 H, m), 2.78–2.70 (3 H, m), 2.59–2.56 (2 H, m), 1.78–1.60 (8 H, m), 0.93 (3 H, t, J = 7.35); 13C-NMR (d4-MeOH): 147.35, 143.27, 143.03, 129.49, 129.12, 127.57, 127.11, 126.91, 79.98, 74.20, 58.38, 54.26, 54.12, 43.51, 38.71, 25.90, 25.79, 22.31, 14.06; LC/MS-MS: 460.23 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-propylphenyl)butan-1-ol (3u). Yield: 69 %; mp: 173 °C; IR: 2959, 2363, 1606, 1446, 1327, 1176, 1071; 1H-NMR (d4-MeOH): 8.52 (1 H, s), 7.51 (4 H, d, J = 7.62), 7.31–7.26 (6 H, m), 7.21–7.16 (4 H, m), 4.65–4.63 (1 H, m), 3.47–3.45 (2 H, m), 3.04–3.01 (2 H, m), 2.94–2.78 (4 H, m), 1.83–1.68 (8 H, m), 1.22 (6 H, t, J = 6.93); 13C-NMR (d4-MeOH): 149.42, 147.18, 143.27, 129.18, 127.67, 127.41, 127.08, 126.97, 79.86, 74.13, 53.97, 53.87, 49.01, 42.97, 37.06, 35.10, 25.52, 24.46, 22.01; LC/MS-MS: 458.13 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-n-butylphenyl)butan-1-ol (3v). Yield: 65 %; mp: 175 °C; IR: 2932, 2366, 1596, 1448, 1342, 1065; 1H-NMR (d4-MeOH): 8.54 (1 H, s), 7.51 (4 H, d, J = 7.48), 7.30–7.24 (6 H, m), 7.18–7.14 (4 H, m), 4.64–4.62 (1 H, m), 3.41–3.35 (2 H, m), 2.93–2.90 (2 H, m), 2.80–2.74 (3 H, m), 2.60–2.57 (2 H, m), 1.81–1.65 (8 H, m), 1.60–1.54 (2 H, m), 1.37–1.32 (2 H, m), 0.92 (3 H, t, J = 7.40); 13C-NMR (d4-MeOH): 147.27, 143.29, 143.18, 129.45, 129.14, 127.61, 127.10, 126.92, 85.09, 79.96, 74.27, 54.08, 43.34, 37.33, 36.27, 35.01, 31.79, 25.81, 23.31, 22.37, 19.23, 14.27; LC/MS-MS: 472.20 (M+H+).
4-(4’-(Hydroxydiphenylmethyl)piperidin-1-yl)-1-(4-iso-butylphenyl)butan-1-ol (3w). Yield: 68 %; mp: 177 °C; IR: 2950, 2361, 1595, 1447, 1328, 1171, 1067; 1H-NMR (d4-MeOH): 8.54 (1 H, s), 7.52–7,50 (4 H, d, J = 7.42), 7.30–7.24 (6 H, m), 7.18–7.15 (2 H, m), 7.13–7.11 (2 H, m), 4.65–4.62 (1 H, m), 3.42–3.38 (2 H, m), 2.96–2.93 (2 H, m), 2.83–2.74 (3 H, m), 2.45 (2 H, d, J = 7.18), 1.86–1.66 (9 H, m), 0.88 (6 H, d, J = 6.63); 13C-NMR (d4-MeOH): 147.29, 143.32, 142.10, 130.18, 129.17, 127.64, 127.12, 126.81, 79.95, 74.27, 58.25, 54.15, 46.12, 43.29, 37.29, 31.52, 25.76, 22.71, 22.32; LC/MS-MS: 472.34 (M+H+).
4-tert-Butylphenyl-6-bromohexanoate (4a). Yield: 61 %; 1H-NMR: 7.41–7.40 (2 H, d, J = 8.59), 7.03–7.02 (2 H, d, J = 8.77), 3.47–3.44 (2 H, t, J = 6.72), 2.61–2.58 (2 H, t, J = 7.38), 1.98–1.92 (2 H, m), 1.84–1.78 (2 H, m), 1.63–1.59 (2 H, m), 1.34 (9 H, s); 13C-NMR: 172.06, 148.59, 148.36, 126.31, 120.84, 77.33, 77.06, 76.82, 34.49, 34.17, 33.43, 32.41, 31.44, 27.64, 24.12.
N-(4-tert-Butylphenyl)-6-bromohexanamide (4b). Yield: 71 %; 1H-NMR: 7.43–7.41 (2 H, d, J = 8.59), 7.34–7.32 (2 H, d, J = 8.58), 3.43–3.40 (2 H, t, J = 6.70), 2.38–2.25 (2 H, t, J = 7.38), 1.93–1.87 (2 H, m), 1.79–1.73 (2 H, m), 1.56–1.50 (2 H, m), 1.30 (9 H, s); 13C-NMR: 170.95, 147.37, 135.09, 125.80, 119.72, 38.74, 37.37, 33.53, 32.43, 31.34, 27.72, 24.70.
4-tert-Butylphenyl-6-(4’-(hydroxydiphenylmethyl)pi-peridin-1-yl)hexanoate (5a). Yield: 52 %; IR: 3398, 2962, 2359, 1749, 1591, 1509, 1448, 1347, 1207, 1172, 1147, 1108, 1017, 951, 839, 749, 702; 1H-NMR: 8.46 (1 H, s), 7.54–7.53 (4 H, m), 7.41–7.39 (2 H, m), 7.30–7.29 (4 H, m), 7.18–7.17 (2 H, m), 6.99–6.98 (2 H, m), 3.50 (2 H, s), 3.04–2.82 (5 H, m), 2.60–2.59 (2 H, m), 1.83–1.70 (8 H, m), 1.47 (2 H, s), 1.30 (9 H, s); 13C-NMR: 173.82, 169.06, 169.02, 149.95, 147.27, 129.25, 127.76, 127.36, 127.12, 122.12, 79.88, 53.77, 42.93, 35.38, 34.68, 31.94, 27.74, 27.14, 25.35, 24.85, 24.41, 24.03, 23.82; LC/MS-MS: 514.25 (M+H+).
N-(4-tert-Butylphenyl)-6-(4-(hydroxydiphenylmethyl)piperidin-1-yl)hexanamide (5b). Yield: 36 %; IR: 3286, 2959, 1662, 1598, 1537, 1448, 1346, 1111, 1031, 953, 839, 749, 702; 1H-NMR: 8.44 (1 H, s), 7.53–7.52 (4 H, d, J = 7.36), 7.46–7.44 (2 H, d, J = 8.70), 7.34–7.28 (6 H, m), 7.19–7.16 (2 H, t, J = 7.35), 3.63 (1 H, s), 3.53–3.51 (2 H, m), 3.07–2.99 (4 H, m), 2.87–2.81 (1 H, m), 2.41–2.38 (2 H, t, J = 7.16), 1.78–1.71 (8 H, m), 1.46–1.43 (2 H, m), 1.30 (9 H, s); 13C-NMR: 174.07, 148.32, 147.13, 129.20, 127.69, 127.11, 126.58, 121.09, 42.88, 42.85, 37.31, 35.25, 31.82, 27.22, 27.18, 26.06, 26.02, 24.90; LC/MS-MS: 513.27 (M+H+).
5-Bromo-1-(4-tert-butylphenyl)hexan-1-ol (6a). Yield: 74 %; 1H-NMR (d4-MeOH): 7.26–7.24 (2 H, d, J = 7.85), 7.13–7.12 (2 H, d, J = 7.81), 4.47–4.44 (1 H, t, J = 6.80), 3.27–3.24 (2 H, t, J = 6.72), 2.43–2.39 (1 H, m), 1.74–1.56 (4 H, m), 1.34–1.33 (4 H, m), 1.22 (9 H, s); 13C-NMR: 150.11, 141.63, 125.41, 125.08, 73.89, 38.49, 34.31, 33.64, 32.54, 31.26, 27.90, 24.86.
1-(5-Bromohexyl)-4-tert-butylbenzene (7a).
To a mixture of indium(III) chloride (33 mg) and 6a (920 mg) in dry dichloromethane chlorodiphenylsilane (1.2 mL) was added dropwise. After stirring at room temperature for 3 hours the solution was hydrolyzed with a little amount of water. Diethyl ether was added, the organic layer separated and the water phase extracted with diethyl ether two times. The combined organic layers were dried, the solvent removed under reduced pressure and the raw product was purified by column chromatography (ethyl acetate/n-hexane: 1/12, followed by pure hexane). Yield: 23 %; 1H-NMR (d4-MeOH): 7.26–7.24 (2 H, d, J = 8.27), 7.06–7.05 (2 H, d, J = 8.26), 3.34–3.31 (2 H, t, J = 6.83), 2.55–2.52 (2 H, t, J = 7.88), 1.82–1.78 (2 H, m), 1.59–1.55 (2 H, m), 1.43–1.38 (2 H, m), 1.34–1.30 (2 H, m), 1.27 (9 H, s); 13C-NMR: 148.33, 139.38, 127.97, 125.08, 35.23, 34.28, 33.78, 32.73, 31.41, 31.18, 28.45, 28.02.
(1-(6-(4-tert-Butylphenyl)hexyl)piperidin-4-yl)diphenylmethanol (8a). Yield: 40 %; IR: 2933, 2363, 1662, 1596, 1491, 1447, 1343, 1172, 1067, 955, 832, 749, 702; 1H-NMR: 7.46–7.45 (4 H, d, J = 8.49), 7.26–7.22 (6 H, t, J = 8.45), 7.14–7.11 (2 H, t, J = 7.31), 7.05–7.03 (2 H, d, J = 8.26), 3.43–3.41 (2 H, d, J = 11.05), 2.81–2.77 (2 H, m), 2.58–2.55 (2 H, m), 2.52–2.49 (2 H, t, J = 7.85), 2.15–2.13 (2 H, m), 1.68–1.67 (2 H, m), 1.58–1.55 (4 H, m), 1.31–1.29 (4 H, m), 1.27 (9 H, s); 13C-NMR: 148.45, 145.48, 139.22, 128.27, 127.96, 126.65, 125.54, 125.12, 78.78, 52.52, 52.5, 52.46, 52.45, 45.38, 42.50, 35.13, 34.30, 31.39, 31.09, 28.72, 26.79, 23.76, 23.31, 23.27; LC/MS-MS: 485.00 (M+H+).
1-(4-iso-Butylphenyl)-5-(piperidin-1-yl)pentan-1-one (9a). Yield: 78 %; mp: 143 °C; IR: 2955, 2869, 1739, 1676, 1604, 1453, 1368, 1273, 1184, 1022; 1H-NMR: 7.93–7.91 (2 H, d, J = 8.31), 7.30–7.28 (2 H, d, J = 8.25), 3.13–3.07 (4 H, m), 2.56–2.55 (2 H, d, J = 7.23), 1.95–1.68 (12 H, m), 1.35–1.32 (1 H, m), 0.92–0.91 (6 H, d, J = 6.63); 13C-NMR: 201.45, 149.18, 136.01, 130.53, 129.21, 64.32, 64.29, 58.04, 54.22, 52.98, 46.31, 38.41, 31.35, 24.60, 24.27, 22.82, 22.69, 22.15, 16.65, 16.60; LC/MS-MS: 302.93 (M+H+).
1-(4-iso-Butylphenyl)-5-(4-methylpiperidin-1-yl)pentan-1-one (9b). Yield: 21 %; IR: 2956, 2361, 1679, 1605, 1457, 1414, 1369, 1336, 1182, 977, 855, 759; 1H-NMR: 7.88–7.86 (2 H, d, J = 8.24), 7.26–7.24 (2 H, d, J = 8.19), 3.52–3.51 (2 H, m), 3.06–3.03 (2 H, t, J = 6.84), 2.99–2.96 (2 H, m), 2.64–2.54 (4 H, m), 1.93–1.75 (9 H, m), 1.63–1.62 (1 H, m), 1.04–1.02 (3 H, d, J = 6.37), 0.93–0.92 (6 H, d, J = 6.62); 13C-NMR: 199.10, 147.76, 134.45, 129.31, 127.96, 45.36, 37.37, 30.07, 23.41, 22.31, 21.19; LC/MS-MS: 315.94 (M+H+).
1-(4-iso-Butylphenyl)-5-(4-hydroxypiperidin-1-yl)-pentan-1-one (9c). Yield: 68 %; IR: 3345, 2954, 2495, 1735, 1679, 1605, 1465, 1414, 1372, 1239, 1181, 1042, 978, 852, 763; 1H-NMR: 7.90–7.88 (2 H, d, J = 8.27), 7.26–7.25 (2 H, d, J = 8.20), 3.93 (1 H, s), 3.38 (2 H, s), 3.19–3.05 (5 H, m), 2.52–2.51 (2 H, d, J = 7.21), 2.06–2.03 (2 H, m), 1.89–1.73 (8 H, m), 0.88–0.87 (6 H, d, J = 6.63); 13C-NMR: 201.44, 149.14, 136.02, 130.54, 129.23, 71.13, 57.71, 46.32, 38.47, 31.83, 31.35, 24.77, 22.72, 22.13, 20.94; LC/MS-MS: 318.11 (M+H+).
5-(4-Benzylpiperidin-1-yl)-1-(4-iso-butylphenyl) pentan-1-one (9d). Yield: 34 %; mp: 143 °C; IR: 3454, 2931, 1682, 1605, 1455, 1241, 1112; 1H-NMR: 7.86–7.84 (2 H, d, J = 8.26), 7.27–7.10 (7 H, m), 7.95–7.92 (2 H, m), 2.86 (2 H, d, J = 11.65), 2.51–2.50 (4 H, d, J = 7.21), 2.33–2.30 (2 H, m), 1.90–1.80 (3 H, m), 1.80–1.69 (2 H, m), 1.61–1.46 (5 H, m), 1.32–1.20 (2 H, m), 0.89–0.88 (6 H, d, J = 6.62); 13C-NMR: 199.89, 147.24, 140.66, 134.76, 129.18, 129.03, 128.05, 127.98, 125.66, 77.01, 70.54, 58.67, 53.90, 45.30, 43.18, 38.25, 37.91, 32.15, 30.02, 26.69, 22.52, 22.27; LC/MS-MS: 392.07 (M+H+).
1-(4-iso-Butylphenyl)-5-(piperidin-1-yl)pentan-1-ol (10a). Yield: 94 %; mp: 64 °C; IR: 3386, 2952, 1587, 1446, 1347, 1018; 1H-NMR (d4-MeOH): 8.55 (1 H, s), 7.25–7.24 (2 H, d, J = 8.01), 7.12–7.10 (2 H, d, J = 8.36), 4.60–4.58 (1 H, m), 3.31–2.98 (3 H, m), 2.85–2.82 (2 H, m), 2.46–2.45 (2 H, d, J = 7.18), 1.87–1.61 (10 H, m), 1.48–1.28 (3 H, m), 0.90–0.88 (2 H, d, J = 6.62); 13C-NMR (d4-MeOH): 170.38, 143.64, 141.89, 130.07, 126.92, 74.61, 58.69, 54.46, 46.13, 31.50, 25.46, 25.43, 24.26, 24.15, 23.40, 22.70; LC/MS-MS: 306.92 (M+H+).
1-(4-iso-Butylphenyl)-5-(4-methylpiperidin-1-yl)pentan-1-ol (10b). Yield: 57 %; IR: 3349, 2953, 2667, 1717, 1594, 1458, 1177, 1051, 948, 848; 1H-NMR (d4-MeOH): 7.23–7.21 (2 H, d, J = 8.01), 7.11–7.09 (2 H, d, J = 7.82), 3.54–3.53 (2 H, m), 2.92–2.90 (2 H, m), 2.56–2.54 (2 H, m), 2.45–2.44 (2 H, d, J = 7.15), 1.87–1.70 (10 H, m), 1.50–1.39 (3 H, m), 1.02–1.01 (3 H, d, J = 6.47), 0.89–0.88 (6 H, d, J = 6.61); 13C-NMR: 141.75, 141.05, 129.21, 125.55, 73.65, 57.35, 52.99, 45.08, 37.98, 30.19, 23.04, 22.36, 20.97; LC/MS-MS: 318.04 (M+H+).
1-(4-iso-Butylphenyl)-5-(4-hydroxypiperidin-1-yl)-pentan-1-ol (10c). Yield: 47 %; IR: 3357, 2950, 2868, 2670, 1466, 1383, 1040, 973, 849, 802, 609; 1H-NMR: 7.28–7.27 (2 H, d, J = 8.01), 7.14–7.13 (2 H, d, J = 8.01), 4.64–4.62 (1 H, t, J = 7.19), 4.10–3.56 (2 H, m), 3.37 (1 H, s), 3.25 (1 H, s), 3.09–3.06 (2 H, t, J = 8.17), 2.48–2.47 (2 H, d, J = 7.17), 2.12–1.77 (10 H, m), 1.52–1.38 (2 H, m), 0.91–0.90 (6 H, d, J = 6.62); 13C-NMR: 143.61, 141.94, 130.10, 126.93, 74.62, 52.21, 46.13, 39.39, 31.51, 30.98, 24.07, 22.72; LC/MS-MS: 319.93 (M+H+).
5-(4-Benzylpiperidin-1-yl)-1-(4-iso-butylphenyl) pentan-1-ol (10d). Yield: 45 %; mp: 64 °C; IR: 2929, 2361, 1454, 1275; 1H-NMR (d4-MeOH): 7.30–7.17 (7 H, m), 7.12–7.10 (2 H, d, J = 7.99), 7.19–7.12 (4 H, m), 4.61–4.59 (1 H, m), 3.50–3.49 (2 H, m), 3.03–2.87 (4 H, m), 2.62–2.61 (2 H, d, J = 7.17), 2.62–2.61 (2 H, m), 1.89–1.71 (8 H, m), 1.52–1.47 (4 H, m), 0.89–0.88 (2 H, d, J = 6.05); 13C-NMR (d4-MeOH): 143.59, 141.95, 140.50, 130.19, 130.10, 129.50, 127.40, 126.91, 74.61, 46.12, 39.36, 31.51, 25.10, 24.07, 22.71; LC/MS-MS: 394.24 (M+H+).
1-(4-iso-Butylphenyl)-5-morpholinopentan-1-one (11a). Yield: 63 %; IR: 2867, 1681, 1606, 1466, 1353, 1251, 1182, 1114, 988, 949, 861, 734; 1H-NMR: 7.85–7.83 (2 H, d, J = 8.21), 7.22–7.20 (2 H, d, J = 8.14), 3.88–3.86 (4 H, t, J = 4.76), 3.00–2.98 (2 H, t, J = 6.22), 2.90 (4 H, s), 2.79–2.76 (2 H, t, J = 7.28), 2.52–2.50 (2 H, d, J = 7.19), 1.89–1.87 (1 H, m), 1.76–1.75 (4 H, m), 0.89–0.88 (6 H, d, J = 6.62); 13C-NMR: 199.68, 147.25, 134.66, 129.14, 127.88, 70.57, 66.73, 58.56, 53.58, 45.24, 38.06, 29.96, 25.97, 22.02, 22.14; LC/MS-MS: 304.12 (M+H+).
1-(4-iso-Butylphenyl)-5-morpholinopentan-1-ol (12a). Yield: 26 %; IR: 3422, 2952, 2868, 2465, 1722, 1599, 1514, 1466, 1366, 1264, 1105, 977, 849, 803, 620 1H-NMR: 7.26–7.24 (2 H, d, J = 8.00), 7.13–7.11 (2 H, d, J = 8.03), 4.62–4.60 (1 H, m), 3.89 (4 H, s), 3.20 (4 H, s), 3.06–3.03 (2 H, t, J = 8.31), 2.47–2.45 (2 H, d, J = 7.18), 1.84–1.74 (5 H, m), 1.49–1.35 (2 H, m), 0.90–0.88 (6 H, d, J = 6.63) 13C-NMR: 143.62, 141.94, 130.10, 126.92, 74.63, 71.51, 65.28, 58.56, 53.25, 46.13, 45.60, 39.43, 31.51, 24.81, 24.00, 22.71 LC/MS-MS: 306.08 (M+H+).
1-(5-(4-iso-Butylphenyl)-5-oxopentyl)piperidin-4-one (13a). Yield: 3 %; IR: 2955, 2869, 2808, 1716, 1678, 1606, 1570, 1466, 1307, 1275, 1183, 954, 846, 796; 1H-NMR: 7.93–7.90 (2 H, m), 7.30–7.28 (2 H, m), 2.79–2.76 (2 H, t, J = 6.16), 2.56–2.42 (9 H, m), 1.93–1.90 (1 H, m), 1.78–1.60 (7 H, m), 0.92–0.91 (6 H, d, J = 6.63); 13C-NMR: 199.42, 129.34, 127.99, 70.48, 56.68, 52.04, 45.41, 39.37, 37.67, 30.04, 22.27, 21.65; LC/MS-MS: 316.15 (M+H+).
5-(3-(Hydroxydiphenylmethyl)pyrrolidin-1-yl)-1-(4-iso-butylphenyl)pentan-1-one (15a). Yield: 36 %; IR: 2957, 1679, 1605, 1448, 1367, 1182, 984, 751, 700; 1H-NMR: 7.79–7.77 (2 H, d, J = 8.15), 7.43–7.40 (4 H, t, J = 8.27), 7.27–7.22 (4 H, m), 7.18–7.14 (4 H, m), 3.75–3.72 (1 H, m), 3.07–2.92 (8 H, m), 2.49–2.47 (2 H, d, J = 7.19), 2.16–1.99 (2 H, m), 1.86–1.84 (1 H, m), 1.72–1.69 (4 H, m), 0.87–0.85 (6 H, d, J = 6.62); 13C-NMR: 199.11, 147.86, 145.41, 134.32, 129.37, 128.65, 128.46, 127.97, 127.91, 127.04, 125.51, 125.45, 78.48, 55.24, 54.54, 45.61, 45.36, 37.28, 30.07, 25.56, 25.13, 22.30, 20.89; LC/MS-MS: 470.10 (M+H+).
5-(3-(Hydroxydiphenylmethyl)pyrrolidin-1-yl)-1-(4-iso-butylphenyl)pentan-1-ol (16a). Yield: 48 %; IR: 3351, 2954, 2373, 1597, 1448, 1338, 1176, 1107, 1067, 1034, 850, 750, 703; 1H-NMR: 7.45–7.42 (4 H, t, J = 7.67), 7.29–7.24 (4 H, m), 7.19–7.16 (4 H, m), 7.08–7.06 (2 H, d, J = 7.97), 4.62–4.59 (1 H, m), 3.77–3.74 (1 H, m), 2.91–2.88 (6 H, m), 2.43–2.42 (2 H, d, J = 7.14), 2.19–2.15 (2 H, m), 1.83–1.79 (1 H, m), 1.76–1.64 (4 H, m), 1.46–1.35 (2 H, m), 0.87–0.86 (6 H, d, J = 6.61); 13C-NMR: 145.66, 141.83, 141.02, 129.20, 128.63, 128.47, 127.19, 127.07, 125.58, 125.52, 125.49, 78.49, 73.66, 61.84, 54.56, 54.38, 45.09, 37.95, 30.20, 25.63, 25.32, 22.91, 22.37; LC/MS-MS: 472.20 (M+H+).
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) for the grants given to Marcel Holzer and Sigrid Ziegler (KFO129) and the Fonds der Chemischen Industrie for financial support. Furthermore we thank Susanne Reinhardt for the syntheses of compounds 9a, 9d, 10a and 10d.
Footnotes
Sample Availability: Contact the authors.
References and Notes
- 1.Chisari F. V. Unscrambling hepatitis C virus-host interactions. Nature. 2005;436:930–932. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- 2.WHO Annex Table 2: deaths by cause, sex and mortality stratum in WHO regions, estimates for 2001. World Health Report. [(accessed 01.2008)]. 2002. www.who.int/entity/whr/2002/en/whr2002_annex2002.pdf.
- 3.VanCompernolle S. E., Wiznycia A. V., Rush J. R., Dhanasekaran M., Baures P. W., Todd S. C. Small molecule inhibition of hepatitis C virus E2 binding to CD81. Virology. 2003;314:371–380. doi: 10.1016/S0042-6822(03)00406-9. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler S., Kronenberger B., Zeuzem S., Hartmann R. W., Klein C. D. in preparation.
- 5.Pileri P., Uematsu Y., Campagnoli S., Galli G., Falugi F., Petracca R., Weiner A. J., Houghton M., Rosa D., Grandi G., Abrignani S. Binding of hepatitis C virus to CD81. Science. 1998;282:938–941. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 6.Carr A., Clyde R. Piperidinderivate. 25.06.770. D.E. Patent. 1975
- 7.Pietschmann T., Kaul A., Koutsoudakis G., Shavinskaya A., Kallis S., Steinmann E., Abid K., Negro F., Dreux M., Cosset F. L., Bartenschlager R. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc. Natl. Acad. Sci. USA. 2006;103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koutsoudakis G., Kaul A., Steinmann E., Kallis S., Lohmann V., Pietschmann T., Bartenschlager R. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J. Virol. 2006;80:5308–5320. doi: 10.1128/JVI.02460-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lohmann V., Korner F., Koch J., Herian U., Theilmann L., Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110–113. doi: 10.1126/science.285.5424.110. [DOI] [PubMed] [Google Scholar]
- 10.Kato T., Date T., Miyamoto M., Furusaka A., Tokushige K., Mizokami M., Wakita T. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology. 2003;125:1808–1817. doi: 10.1053/j.gastro.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Zhang W. C., Li C. J. A direct retro-barbier fragmentation. J. Org. Chem. 2000;65:5831–5833. doi: 10.1021/jo000129r. [DOI] [PubMed] [Google Scholar]
- 12.Kawakita T., Kuroita T., Murozono T., Hakira H., Haga H., Ito K., Sonda S., Kawahara S., Asona K. Benzoic acid compounds and use thereof as medicaments. 5,864,039,1999. U.S. Patent. 1999
- 13.Wagner P. J., Lindstrom M. J., Sedon J. H., Ward D. R. Photochemistry of & Halo Ketones: Anchimeric Assistance in Triplet-State g-Hydrogen Abstraction and b-Elimination of Halogen Atoms from the Resulting Diradicals. J. Am. Chem. Soc. 1981;103:3842–3849. doi: 10.1021/ja00403a036. [DOI] [Google Scholar]
- 14.Zhang M. Q., ter Laak A. M., Timmerman H. Structure-activity relationships within a series of analogues of the histamine H1-antagonist terfenadine. Eur. J. Med. Chem. 1993;28:165–173. doi: 10.1016/0223-5234(93)90009-4. [DOI] [Google Scholar]
- 15.Lafite P., Andre F., Zeldin D. C., Dansette P. M., Mansuy D. Unusual regioselectivity and active site topology of human cytochrome P450 2J2. Biochemistry. 2007;46:10237–10247. doi: 10.1021/bi700876a. [DOI] [PMC free article] [PubMed] [Google Scholar]