Abstract
Background:
Skeletal related events (SREs), common sequelae of metastatic cancer, are reduced by bisphosphonates. In this study, it was postulated that radiopharmaceuticals, added to bisphosphonates could further decrease the incidence of SREs.
Methods:
NRG Oncology RTOG 0517 randomized patients with breast, lung and prostate cancer and blastic bone metastases to either Zoledronic acid (ZA) alone or ZA plus radiopharmaceuticals (Sr-89 or Sm-153). The primary endpoint was time to development of SREs. Secondary objectives included quality of life (QOL), pain control, overall survival (OS) and toxicity.
Results:
261 patients (median age 68; 62% male; 55% prostate, 35% breast, 10% lung) were accrued between July 2006 and February 2011. The study closed early due to a lower than expected rate of SREs. 52 (42%) patients in the ZA arm and 49 (40%) in the radiopharmaceutical arm experienced an SRE. Median time free of SREs was 29.9 and 27.4 months, respectively (p=0.84). Median OS in the ZA arm and radiopharmaceutical arms was 32.1 and 26.9 months, respectively (p=0.37). Cox proportional hazards regression model showed that primary disease site (lung) and number of bone metastases (>2) had a negative impact on OS (p<0.0001, p=0.01, respectively). The addition of radiopharmaceuticals to ZA led to a significant reduction in pain at one month based on BPI worst score (p=0.02). No other group differences were noted for QOL or toxicity.
Conclusion:
The addition of radiopharmaceuticals to bisphosphonates did not alter time to SREs or OS for patients with breast, lung, prostate cancers and blastic bone metastases; although it was associated with significant pain reduction at one month.
Clinical Trial Registry:
This protocol (RTOG 0517) is registered with ClinicalTrials.gov (NCT00365105), and may be viewed online at http://www.clinicaltrials.gov/ct2/show/NCT00365105?term=RTOG+0517&rank=1.
Introduction
Each year, approximately 400,000 cancer patients in the U.S. develop bone metastases (1). Cancer spreading to the bone is associated with skeletal related events (SREs) defined as bone pain, fracture, spinal cord compression needing other intervention, or hypercalcemia. Breast, prostate and lung cancers metastasize to bone most frequently (2) and the incidence of bone metastasis is expected to increase over the next decade as patient survival improves. Therapy for bone metastases is palliative. Syndromes related to bone metastases include pain, impaired mobility, decreased bone marrow function, fractures, hypercalcemia and spinal cord compression (3). Treatment often does not start until the patient presents with symptoms, at which point multiple therapeutic options exist including pain medication, radiation therapy, radiopharmaceuticals, orthopedic stabilization, bone modifying agents including bisphosphonates and Denosumab, hormonal manipulation and systemic chemotherapy (4). As such, the ultimate goal for the management of bone metastases is to relieve pain and to improve physiologic function while preventing more serious complications from disease progression.
Bone remodeling is a balance between osteoblastic and osteoclastic activities (5) which are controlled by various substances including tumor necrosis factors, interleukins-1 and 6, prostaglandins, vitamins and cytokines. Studies have shown that osteoblasts control osteoclast activity through RANKL ligand as well as other molecular pathways (6). Tumor cells that disrupt these processes can precipitate destruction of bone.
While any of the above treatments may be effective in management of complications caused by bone metastases, bisphosphonates and RANKL inhibitors are the only agents explicitly approved for use in reducing SREs. They may also lead to a general slowing or reversal of the progression of skeletal metastases (7). It is unknown whether radioisotopes in asymptomatic patients delay the time to disease progression or the rate of development of SREs.
The primary goal of NRG Oncology RTOG 0517 was to determine if the addition of a radioisotope, (Strontium-89 [SR-89] or Samarium-153 [Sm-153]), to bisphosphonates for patients with asymptomatic or stable symptomatic bone metastasis could delay the time to development of malignant skeletal related events (SREs). Secondary goals included assessment of overall survival (OS), toxicity, and quality of life (QOL) in patients receiving combined treatment versus bisphosphonates alone.
Materials and Methods
Patient Characteristics
NRG Oncology RTOG 0517 was a randomized phase III trial registered with the National Cancer Institute, monitored by the Radiation Therapy Oncology Group Data Monitoring Committee (DMC), and approved by the institutional review boards of participating centers. All patients provided written consent for enrollment. Eligibility included histologically proven cancer of the prostate, breast or lung with confirmed osteoblastic bone metastases (that is, active on a bone scan but not necessarily osteoblastic on x-ray imaging) and no current symptoms or stable pain from their bone disease. Stable pain was defined as a previous site of painful bony disease treated successfully (for example, with radiation therapy) at least two weeks prior to registration and not requiring further intervention. A positive bone scan obtained four weeks prior to study entry was required. Patients had to have adequate bone marrow function to permit radioisotope administration and adequate renal function to permit zoledronic acid (ZA) infusion. Dental evaluation and clearance was also required. ECOG performance status had to be 2 or better for prostate and breast cancer patients but it was limited to 0 or 1 for lung cancer patients. This was because of the shorter length of survival reported in lung cancer patients with poor performance status (8).
Study Design
Patients were stratified by site of primary cancer (lung vs. breast, vs. prostate) and number of bone mets (≤2 vs. > 2) and randomized 1:1 using a treatment allocation scheme devised by Zelen et al (9). Patients in both arms received ZA 4 mg IV monthly. Patients in Arm 1 received ZA alone (ZA), while those in Arm 2 received a single injection of either 4 mCi Sr-89 or 1 mg/kg body weight of Sm-153 and ZA (ZA+R). Radioisotope choice was left to the discretion of the investigator. All patients received supplemental Vitamin D and Calcium. Patients were required to receive their radioisotope within six weeks of randomization. If patients required external beam radiation (EBRT), then all treatment had to be completed two weeks prior to radioisotope infusion, and if EBRT was required after radioisotope treatment for a non-SRE related event, it could not commence for at least two weeks.
Patients receiving systemic chemotherapy or hormonal therapy were eligible for this study, but in patients randomized to the radioisotope arm, chemotherapy had to be withheld two weeks prior to and two weeks following radioisotope infusion. Patients receiving hormonal therapy could not have alterations in the administration schedule of the hormone for the four week period surrounding the radioisotope infusion. Patients in the ZA only arm had no restrictions in the administration time of chemotherapy or hormonal therapy, which was administered at the discretion of the investigator. Patients with prior bisphosphonate use were also eligible for participation as long as the total time on medication was less than six months prior to randomization.
Patients in Arm 2 (radioisotopes) had their complete blood counts including absolute neutrophil count (ANC) assessed weekly for eight weeks following infusion of radioisotope. All patients were required to have a monthly creatinine and a quarterly dental exam performed while on ZA. Patients could be withdrawn from the study for unacceptable adverse events or in the patient’s interest at the discretion of the treating physician.
Study Endpoints
The primary objective was to determine if the addition of a radionucleotide (either Sr-89 or Sm-153) to bisphosphonates for patients with non-painful or stable symptomatic bone metastases could delay the time to development of malignant skeletal related events (SREs). Small et al (10) demonstrated the median time to the first SRE to be 14.8 months (yearly SRE hazard rate = 0.5620) for prostate cancer patients and Domcheck et al (11) estimated 12 months (yearly SRE hazard rate = 0.6932) for breast cancer patients receiving bisphosphonates. Rosen et al (12) demonstrated the median time to the first SRE to be 5.6 months (yearly SRE hazard rate =1.5) for lung cancer patients receiving bisphosphonates. It was expected that the distribution between prostate, breast, and lung cancer patients would be 40%, 40%, and 20%, respectively. Therefore, the weighted yearly SRE hazard rate for patients treated with bisphosphonates was projected to be 0.8 which translated to a median time to SRE of 10.4 months, assuming an exponential distribution. The study was designed to show a 33% relative reduction in the yearly SRE hazard rate resulting in 15.6 months median time to SRE in the Zoledronic acid plus radioisotope arm (ZA+R). Using a two-sided log-rank test, assuming an overall type I error of 0.05, and statistical power of 90%, 257 SREs were required with a total of 316 patients (13). The study sample size was increased by 10% to guard against ineligible cases, resulting in a target accrual of 352 patients. One interim analysis was planned after 128 SREs were reported.
Secondary objectives included determining the rate of SREs over a one year period, OS, QOL, and pain control in both groups. QOL was measured by the Functional Assessment of Cancer Therapy – General (FACT-G), while pain control was measured by the Brief Pain Inventory (BPI). Collection time points occurred prior to initiation of treatment and at 1, 3, 6, and 12 months after initiation of treatment. Adverse events (AEs) were graded using the NCI’s Common Terminology Criteria for Adverse Events (CTCAE), version 3.0.
Statistical Analysis
The time to treatment failure was measured from the date of randomization and compared using a stratified log-rank test with a two-sided significance level of 0.05. Time free of an SRE was estimated using the Kaplan-Meier method and censoring deaths. One-year SRE rates and AE rates were tested using a two-sided Fisher’s exact test. OS was estimated using the Kaplan-Meier method, and a two-sided stratified log-rank test was used to test the difference between treatment arms.
Differences in QOL and pain control between treatment arms were examined using the mean FACT-G scores (total score as well as the four subscale scores) and mean BPI score from baseline to each follow-up assessment time. These differences were tested using a two-sided Wilcoxon rank sum test with a significance level of 0.05. For all analyses using the FACT-G, a Bonferroni-adjusted significance level of 0.01 was used to account for multiplicity. SAS/STAT® v.9.2 software was used for all analyses.
Results
Patient Characteristics
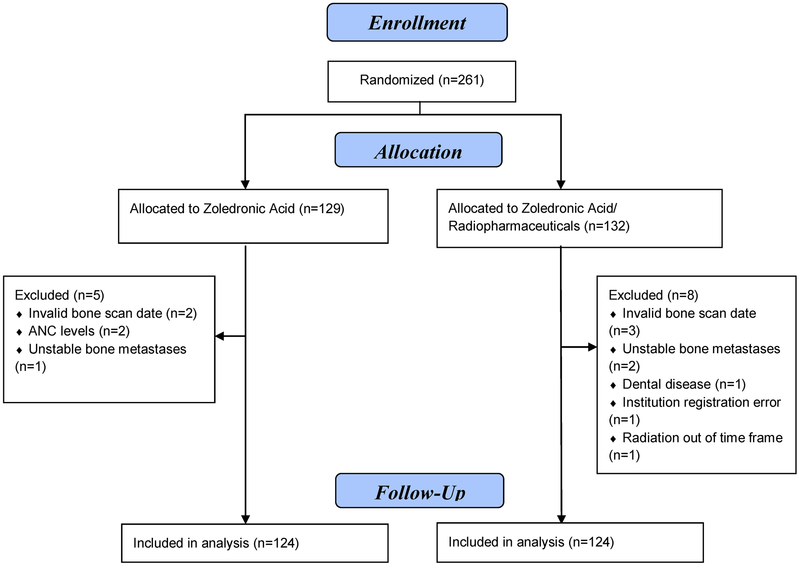
A total of 261 patients were randomized between July 2006 and February 2011 to either ZA or ZA+R. Thirteen patients proved ineligible resulting in 248 patients for the analysis (Figure 1). By the recommendation of the RTOG DMS, the study closed early due to a lower than expected rate of SREs in the control arm. With regard to pretreatment characteristics in the two treatment arms, there were no significant differences with respect to age, gender, race, underlying malignancy, Zubrod status or sites of bone metastases (Table 1). The majority of patients in this study had prostate cancer (55%) followed by breast cancer (35%) then lung cancer (10%). Given the actual disease site distributions, the yearly SRE hazard would have been 0.7 rather than the hypothesized 0.8.
Figure 1.
CONSORT diagram
Table 1.
Pretreatment Characteristics
| ZA (n=124) |
ZA+R (n=124) |
|
|---|---|---|
| Age (years) | ||
| Median | 67.5 | 68 |
| Min - Max | 25–88 | 32–90 |
| Q1–Q3 | 60–77 | 60 – 75.5 |
| Gender | ||
| Male | 77 (62.1%) | 76 (61.3%) |
| Female | 47 (37.9%) | 48 (38.7%) |
| Race | ||
| American Indian/Alaska Native | 1 (0.8%) | 1 (0.8%) |
| Black or African American | 12 (9.7%) | 13 (10.5%) |
| White | 111 (89.5%) | 110 (88.7%) |
| Ethnicity | ||
| Hispanic or Latino | 5 (4.0%) | 4 (3.2%) |
| Not Hispanic or Latino | 113 (91.1%) | 119 (96.0%) |
| Unknown (Individuals not reporting ethnicity) | 6 (4.8%) | 1 (0.8%) |
| Zubrod Performance Status | ||
| 0 | 62 (50.0%) | 73 (58.9%) |
| 1 | 53 (42.7%) | 47 (37.9%) |
| 2 | 9 (7.3%) | 4 (3.2%) |
| Primary Disease Site (stratification factor) | ||
| Breast | 42 (33.9%) | 44 (35.5%) |
| Lung | 14 (11.3%) | 12 (9.7%) |
| Prostate | 68 (54.8%) | 68 (54.8%) |
| Number of bone metastases (stratification factor) | ||
| <=2 | 27 (21.8%) | 26 (21.0%) |
| >2 | 97 (78.2%) | 98 (79.0%) |
| Site(s) of bone metastases§ | ||
| Sternum | 32 (25.8%) | 29 (23.4%) |
| Spine | 95 (76.6%) | 95 (76.6%) |
| Girdle | 30 (24.2%) | 28 (22.6%) |
| Ribs | 81 (65.3%) | 81 (65.3%) |
| Extremity | 36 (29.0%) | 25 (20.2%) |
| Pubis | 13 (10.5%) | 7 (5.6%) |
| Sacrum | 50 (40.3%) | 42 (33.9%) |
| Pelvis | 56 (45.2%) | 53 (42.7%) |
| Hip | 32 (25.8%) | 35 (28.2%) |
| Femur | 42 (33.9%) | 45 (36.3%) |
| Lower Leg | 5 (4.0%) | 8 (6.5%) |
| Other | 39 (31.5%) | 48 (38.7%) |
| Number of Prior Therapies | ||
| 0 | 13 (10.5%) | 9 (7.3%) |
| 1 | 20 (16.1%) | 22 (17.7%) |
| 2 | 31 (25.0%) | 37 (29.8%) |
| 3 | 36 (29.0%) | 29 (23.3%) |
| 4 | 22 (17.7%) | 23 (18.5%) |
| 5 | 2 (1.6%) | 3 (2.4%) |
| 6 | 0 (0.0%) | 1 (0.8%) |
Q1-first quartile; Q3-third quartile]
Multiple sites allowed
Treatment Results
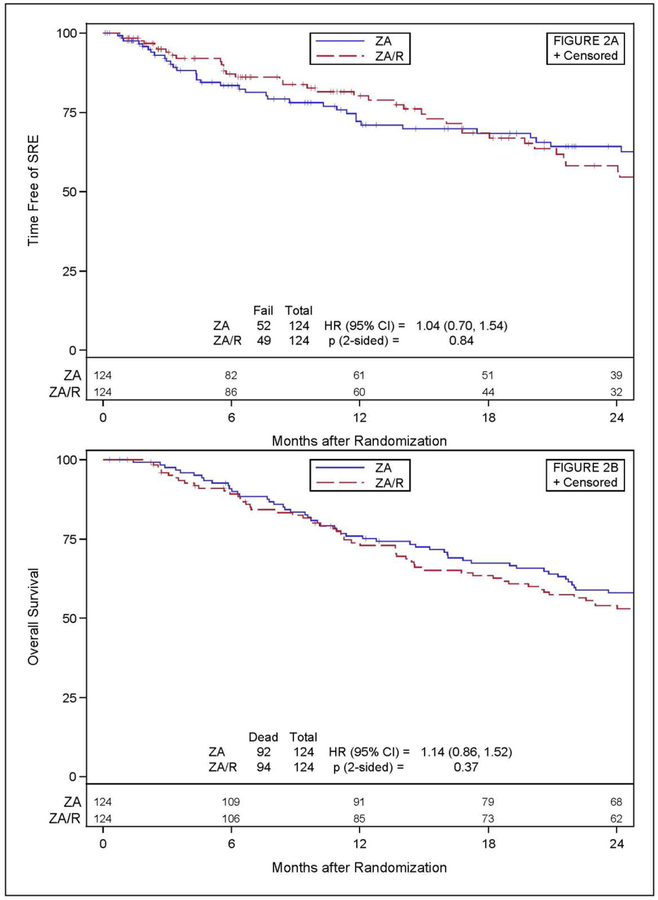
Out of the 248 patients with follow-up information, 52 patients (41.9%) in the ZA arm and 49 (39.5%) in the ZA+R arm experienced a SRE (Table 2). Radiation therapy to new sites of painful bone disease constituted the majority of SREs (73.1% in the ZA arm, 83.7% in the ZA+R arm). Twenty-two patients (14 in the ZA arm and 8 in the ZA+R arm) experienced a pathologic bone fracture, spinal cord compression, or surgery to the bone as the initial SRE. Figure 2a shows time free of an SRE. There was no significant difference between arms (hazard ratio [HR]=1.04, 95% confidence interval [CI]:0.70–1.54, p=0.84). Amongst those who developed an SRE, the median times to first SRE were 29.9 (95% CI: 25.8, 37.4) months for ZA arm and 27.4 (95% CI: 21.2, 35.4) months for ZA+R arm. One year SRE rates between the ZA and ZA+R arms were not different for all patients (22.6% vs. 16.1%, respectively, p=0.26) or for breast (14.3% vs. 18.2%, respectively, p=0.77) or prostate cancer patients (27.9% vs. 13.2%, respectively, p=0.06). The sample size for lung cancer patients was too small to reliably test the between arm difference (n=26). There was no difference in OS between the ZA and ZA+R arms (HR= 1.14, 95% CI:0.86–1.52, p=0.37), as seen in Figure 2b. Additionally, examination of each disease site individually (breast, lung, and prostate) showed no significant differences in OS between the treatment arms for breast and prostate (HR= 1.34, 95% CI:0.78–2.28, p=0.29 and HR=1.02, 95% CI: 0.70–1.49, p=0.50, respectively). OS in the lung cancer population was not assessed due to the small sample size. An adjusted Cox proportional hazards regression model showed that primary disease site and number of bone metastases had a significant impact on overall survival. Specifically, patients with lung cancer and those with more than two bone metastases had a higher risk of death than those with breast or prostate cancer or ≤ two sites of disease (Table 3).
Table 2.
SRE Status
| ZA (n=124) |
ZA+R (n=124) |
|
|---|---|---|
| SRE | 52 (41.9%) | 49 (39.5%) |
| Pathologic Bone Fracture | 7 (13.7%) | 2 (4.1%) |
| Spinal Cord Compression | 2 (3.8%) | 2 (4.1%) |
| Surgery to Bone | 5 (9.8%) | 4 (8.2%) |
| RT to Bone | 38 (74.5%) | 41 (83.7%) |
| Alive, no SRE | 20 (16.1%) | 18 (14.5%) |
| Death prior to SRE | 52 (41.9%) | 57 (46.0%) |
Figure 2.
A) Time free of SREs, B) Overall Survival
Table 3.
Cox Proportional Hazards Model Overall Survival
| Effect | Parameter Estimate | P-value | Hazard Ratio† | 95% Confidence Intervals |
|---|---|---|---|---|
| RX (ZA+R) | −0.24 | 0.11 | 0.78 | [0.58, 1.06] |
| Primary Disease Site (Breast)§ | −0.44 | 0.01 | 0.64 | [0.46, 0.90] |
| Primary Disease Site (Lung)§ | 1.53 | <.0001 | 4.63 | [2.88, 7.44] |
| Number of Bone Metastases (> 2) | 0.48 | 0.01 | 1.62 | [1.13,2.32] |
Reference group is prostate cancer; breast cancer patients had 43 deaths out of 86 patients and lung cancer patients had 25 deaths out of 26 patients.
Interpretation: if hazard ratio > 1, then category in parentheses has a higher hazard than the reference group (omitted category); if hazard ratio < 1, then category in parentheses has a smaller hazard than the reference group (omitted category)
Radiopharmaceuticals were well tolerated (Online Resource Table 1). Of note, patients experiencing a grade 3 or 4 myelosuppression observed across all three cell lines was significantly higher in the ZA+R arm compared to the ZA arm (18% vs. 5%, p=0.0021). There was no increase in gastrointestinal or constitutional toxicity in those receiving radiopharmaceuticals. There were no differences in grade 5 (fatal) adverse events (AEs) reported in either arm (12 in each arm). None of the grade 5 AEs were attributable to therapy.
Approximately 90% of patients consented to participate in QOL and 99% of evaluable patients completed the FACT-G and BPI forms at baseline. Compliance decreased during the course of treatment to 64% at six months and 54% at 12 months for both arms. At 12 months, there was no difference between the arms with respect to FACT-G or BPI results. The only significant difference between the two arms occurred with the change from baseline to one month for BPI worst pain score. Patients on the ZA+R arm had less pain (median of 0) than those on the ZA arm (median of 1), with p=0.02. However, this difference was no longer apparent during subsequent follow-up collection times.
Discussion
The role of bone seeking radionuclides in the treatment of painful bony metastases has been well defined over the past several decades, but its use remains generally limited to near terminal patients whose disease has progressed on virtually all other forms of treatment. Until recently there has been decreasing use of radioisotopes for bone metastases. This is now changing with the use of Ra-223 for prostate cancer patients with bone metastases. The current trial was implemented prior to the FDA approval of Ra-223.
There have been attempts to expand the role of the prior standard radionuclides, Sr-89 and Sm153. In an effort to improve pain control and overall survival in castrate-resistant metastatic prostate cancer patients, several trials featuring Sr-89 and concomitant chemotherapy have been reported (14–16). Randomized trials have compared external beam radiation to Sr-89 and have suggested that radioisotopes can reduce the development of new bone lesions (17,18). This NRG Oncology study was designed to expand on this finding by utilizing radioisotopes to prevent or delay the development of complications of bone metastases, specifically skeletal related events.
The patient population in this study was somewhat different from previous trials in that blastic metastases from three different primary disease sites (prostate, breast and lung) were included. Since the broad consequences of metastatic bone involvement were being studied, it was felt that a more heterogeneous population of patients should be evaluated. However, one unexpected observation was that the rate of SREs was much lower than reported in previous trials. The reason for this discrepancy remains unclear, but there are several possibilities. Patients were allowed to resume other treatments including chemotherapy and hormonal therapy two weeks after the administration of the radioisotope, thereby avoiding delays in palliative chemotherapy and potentially delaying the onset of new SREs. Since chemotherapy drugs were not monitored, newer and more effective agents may have been used. Another possibility is that our patients had a much higher utilization of radiation therapy when compared to enrollees on prior studies. For example, in Rosen’s randomized study, the percentage of patients in either the zoledronic acid or the placebo group receiving XRT to bone as the basis of an SRE ranged from 27–32% of all SREs (12). In contrast, more than 90% of our patients received XRT for an SRE in either the ZA or ZA+R groups prior to enrollment; so it is possible that XRT had already accomplished significant palliation. While it is true that Rosen’s study excluded breast and prostate patients who may have had fewer surgical SREs, it is still not unreasonable to postulate that differences in SRE rates could have been obscured by high early radiotherapy utilization rates. In addition, there were 15% more prostate cancer patients enrolled in NRG Oncology RTOG 0517 than hypothesized. Since the time to SRE differs for each type of cancer, the hypothesized enrollment for each site played a large role in determining the hypothesized SRE rate in the control arm. Finally, the mixing of diseases may have been confounding. Most of the previous studies in this area have featured more homogenous patient populations. A case in point, the newest study evaluating radium-223 conducted on castrate-resistant prostate cancer patients showed significantly positive results with respect to both SREs and overall survival (19).
The primary role for radioisotopes has been the treatment of painful bone lesions. However, there have been attempts to use radioisotopes in other ways. Several studies have evaluated concomitant chemotherapy and radioisotopes in an attempt to better control metastatic prostate cancer symptoms and disease progression (20–22). Small phase I and II studies have shown that one could safely deliver chemotherapy and Sr-89 without significant toxicity, and that the combination regimen was effective in reducing both pain and PSA. NRG Oncology RTOG 0517 did not directly involve comparisons of chemotherapy and radioisotopes. However, all enrollees were allowed to return to their normal treatment schedule after the study protocol. This included patients in both arms of the study, presumably eliminating any inadvertent bias. Although the study failed to achieve its primary endpoint, it is important to note that neither arm (ZA nor ZA+R), featured unusual levels of toxicity. This observation would confirm previous findings that one can treat patients undergoing chemotherapy with a radioisotope safely.
In summary, the failure of this study to demonstrate a significant benefit of radiopharmaceuticals was more likely due to the lower than anticipated incidence of SREs in this population, and may have been exacerbated by the lack of a homogeneous population of patients experiencing bone metastasis. Newer radioisotopes such as Radium 223 and newer bone agents such as denosumab need to be evaluated in this setting. However, a successful study would likely require a substantially larger sample size in a more homogenous population with relatively less prior exposure to palliative XRT.
Supplementary Material
Acknowledgements
Funding: This project was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). Additional support was provided by Novartis. This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or of Novartis.
This project was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). Additional support was provided by Novartis. This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or of Novartis.
References
- 1.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Review Cancer 2002;2:584–593 [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J. Cancer 1987;55:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu HH, Tsai YY et al. Overview of diagnosis and management of metastatic disease to bone. Cancer Control 2012;8:84–91 [DOI] [PubMed] [Google Scholar]
- 4.Buga S, Sarria JE. The management of pain in metastatic bone disease Cancer Control 2012;8:154–166 [DOI] [PubMed] [Google Scholar]
- 5.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004. April 15;350(16):1655–64. [DOI] [PubMed] [Google Scholar]
- 6.Bussard KM, Gay CV et al. The bone microenvironment in metastasis: what is special about bone? Cancer Metastasis Rev 2008;27:41–55 [DOI] [PubMed] [Google Scholar]
- 7.Stopeck AT, Lipton A et al. Denosumab compared with Zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized double-blind study. J Clin Oncol 2010;28:5132–5139 [DOI] [PubMed] [Google Scholar]
- 8.West HJ. Patients with advanced Non–Small-cell lung cancer and marginal performance status: Walking the tight rope towards improved survival. J Clin Oncol. 2013;31:2841–2843. [DOI] [PubMed] [Google Scholar]
- 9.Zelen M The randomization and stratification of patients to clinical trials. J Chron Dis. 27: 365–375, 1974. [DOI] [PubMed] [Google Scholar]
- 10.Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 21(23): 4277–84, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Domcheck SM, Younger J, Finkelstein DM et al. Predictors of Skeletal Complications in Patients with Metastatic Breast Carcinoma. Cancer. 89:363–368, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Rosen LS, Gordon D, Tchekmedyian R, Hirsh V, et al. Zoledronic acid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: Phase III, double blind, randomized trial—the Zoledronic Acid Lung Cancer and Other Solid Tumors Study Group. J. Clin Oncol 21(16): 3150–3157, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RE. Skeletal complications of malignancy. Cancer. 80(Suppl 8):1588–1594, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Sciuto R, Festa A et al. Radiosensitization with low-dose carboplatin enhances pain palliation in radioisotope therapy with strontium-89. Nucl Med Commun 1996;17:799–804 [DOI] [PubMed] [Google Scholar]
- 15.Sciuto R, Festa A et al. Effects of low dose cisplatin on 89Sr therapy for painful bony metastases from prostate cancer: a randomized clinical trial. J Nucl Med 2002;43:79–86 [PubMed] [Google Scholar]
- 16.Tu SM, Millikan RE et al. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate. A randomized phase II trial. Lancet 2001;357:336–341 [DOI] [PubMed] [Google Scholar]
- 17.Oosterhof GO, Roberts JT et al. Strontium (89) chloride versus palliative local field radiotherapy in patients with hormonal escaped prostate cancer: a phase III study of the European Organization for Research and treatment of Cancer, Genitourinary Group. Eur Urol 2003;44:519–526 [DOI] [PubMed] [Google Scholar]
- 18.Quilty PM, Kirk D et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol 1994;31:33–40 [DOI] [PubMed] [Google Scholar]
- 19.Parker C, Nilsson S, et al. ALSYMPCA Investigators. Alpha emitter radium-223and survival in metastatic prostate cancer. N Engl J Med. 2013. July 18;369(3):213–23. [DOI] [PubMed] [Google Scholar]
- 20.Morris MJ, Pandit-Taskar N et al. Phase I study of samarium-153 lexidronam with Docetaxel in castration-resistant metastatic prostate cancer. J Clin Oncol 2009;27:2436–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tu SM, Mathew P et al. Phase I study of concurrent weekly Docetaxel and repeated samarium-153 lexidronam in patients with castration-resistant metastatic prostate cancer. J Clin Oncol 2009;27:3319–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fizazi K, Beuzeboc P et al. Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol. 2009. May 20;27 (15):2429–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.