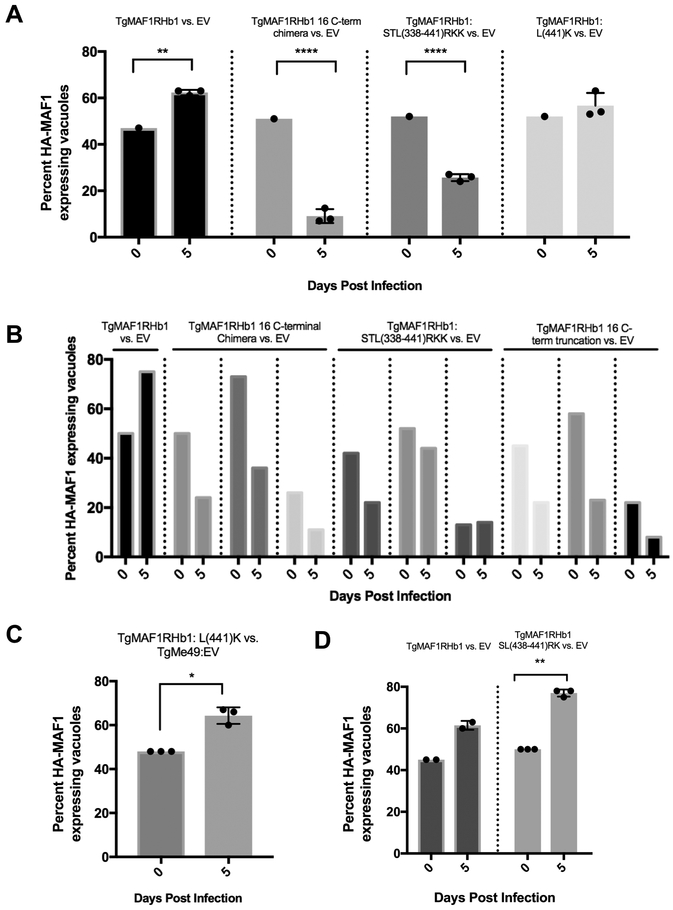
Figure 7. HMA-functional paralog, TgMAF1RHb1 confers in vivo competitive advantage but TgMAF1RHb1 C-terminal mutants do not.
(A) Three mice per control or mutant were infected intraperitoneally (IP) with 50/50 mix of TgMe49 parasites expressing either WT or C-terminal TgMAF1RHb1 mutants and TgMe49:EV parasites (i.e. 50% TgMe49:TgMAF1 16 C-terminal chimera vs. 50% TgMe49 empty vector). HFFs were also infected with each input mixed population, fixed at 18 hpi and visualized utilizing epi-fluorescent microscopy. Immunofluorescence staining was performed with antibodies against the HA epitope tag. Following a five day in vivo infection, extracted peritoneal content was used to infect a monolayer of HFFs. Cells were fixed at 18 hpi and visualized utilizing epi-fluorescent microscopy. Immunofluorescence staining was performed with antibodies against the HA epitope tag. Both input (0 dpi) and output (5 dpi) populations were quantified by measuring the percent of MAF1-HA expressing parasites. **p=0.0038, ****p<0.0001 two-way ANOVA (Sidak test). (B) Similar procedure as A, however input parasites populations were mixes of 25/75, 50/50, and 75/25 and only one mouse was infected for each of the mixes. (C) Similar procedure to A and B. Three mice were IP infected with a natural mixed population of TgMe49:TgMAF1RHb1:L(441)K parasites. HFFs were infected with both input populations and peritoneal population after a five day infection. Cells were fixed and probed for parasites expressing HA-epitope tag by IFA (Paired t-test, *p=0.0174). (D) Similar procedure to A and B. Three mice were IP infected with a 50/50 mixture of TgMe49:EV and TgMe49:TgMAF1RHb1:SL(438/441)RK clones. HFFs were infected with both input populations and peritoneal population after a five day infection. Cells were fixed and probed for parasites expressing HA-epitope tag by IFA (Paired t-test, **p=0.0014).