Figure 3. The SMIRKS chemical query language enables direct chemical perception by substructure matching.
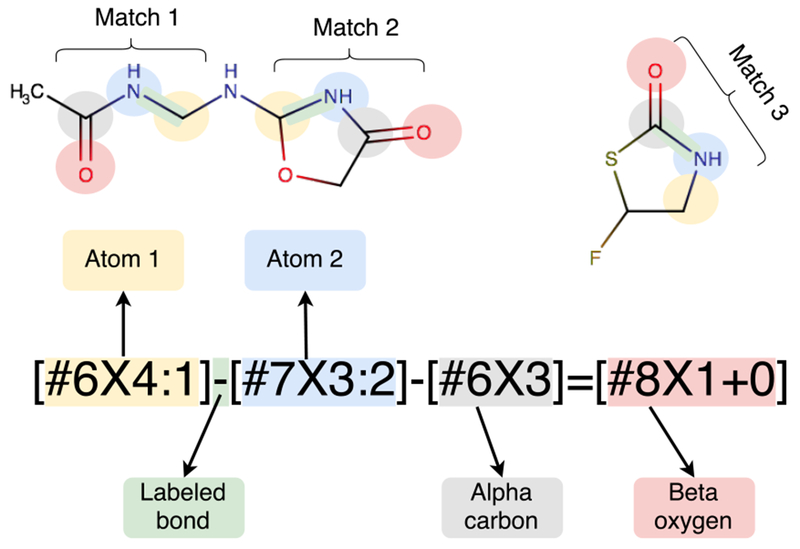
Here, a SMIRKS pattern recognizes the carbon-nitrogen bond in an amide group, so that a bond-stretch parameter can be assigned to it. In the examples, the pattern finds three matches (Matches 1, 2 and 3) in in two different molecules, as indicated by color coding of the atoms and SMIRKS patterns. The relevant pattern is a carbon with four connections ([#6X4:1] (yellow) single bonded (−) to a trivalent nitrogen ([#7X3:2], blue) which is single-bonded to a trivalent carbon ([#6X3], gray) which itself is double bonded (=) to a neutral oxygen with a single connected atom (#8X1+0], red). The first carbon and nitrogen in the pattern are singled out for special treatment by having numerical atom labels (:1 and :2) assigned to them, because the SMIRKS pattern in this case is used to assign a bond parameter to the bond connecting the two labeled atoms.
