Fig. 1.
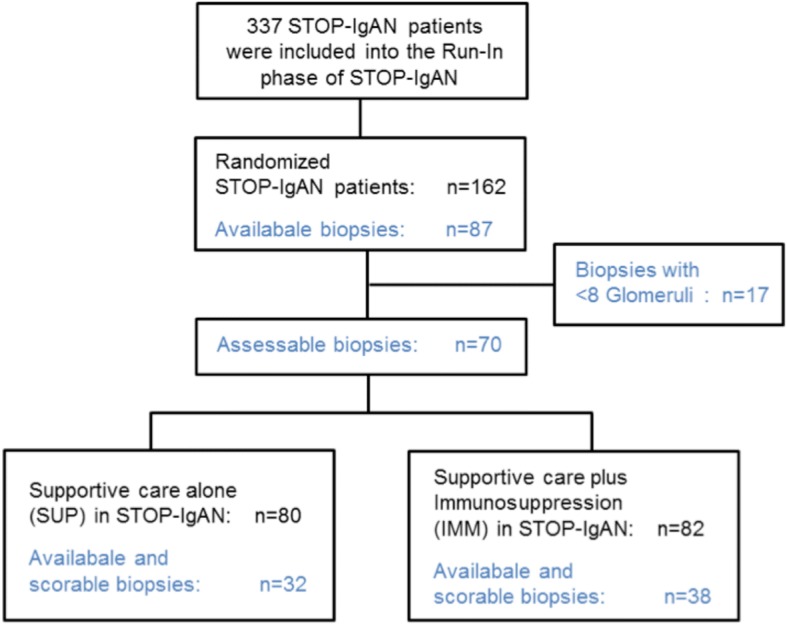
Flowchart of analyzed patients. A total of 337 patients with biopsy-proven IgAN entered the run-in phase of the STOP-IgAN trial during which all patients received supportive care. After 6 months, 162 patients were randomized to either continue on supportive care (n = 80) or received additional immunosuppression (n = 82). Upon amendment of the initial trial protocol in 2009, we aimed to retrieve the original kidney biopsies from the randomized patients for the current secondary analysis. Eventually, 70 biopsies were collected and could be scored using the MEST-C criteria
