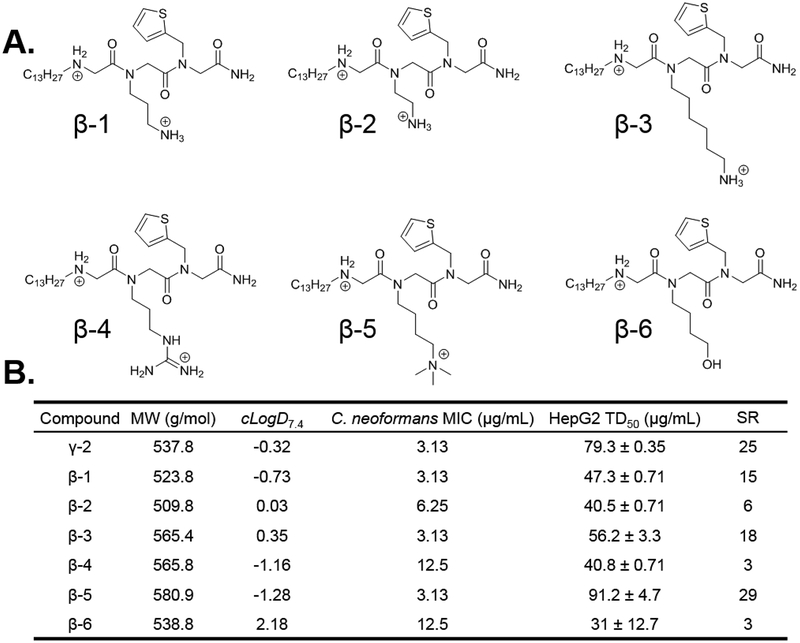
Figure 5.
(A.) Round 3 SAR compounds utilizing the tridecyl tail from Round 1 and the thiophene aromatic heterocyle from Round 2 with varied cationic moieties in position 2. (B.) Calculated distribution coefficient (cLogD7.4), C. neoformans antifungal potency (MIC), and HepG2 liver cell toxicity (TD50) for round 3 SAR compounds. MW = molecular weight; cLogD7.4 = calculated distribution coefficient at pH 7.4; MIC = minimum inhibitory concentration; TD50 = toxic dose 50%; SR = selectivity ratio (TD50/MIC).