Abstract
Postoperative chemotherapy has been widely used in the treatment of early‐staged ovarian cancer patients underwent unilateral resection, but the clinical decision mainly depends on the doctor’s experience without a well‐defined guideline. This study used propensity score matching to analyze the effect of postoperative chemotherapy for early‐staged ovarian cancer patients underwent unilateral resection on prognosis. Patients of age 50 or younger than 50 with early‐staged ovarian cancer were explored from the Surveillance, Epidemiology, and End Results program database during 2000‐2018. Propensity score matching was used to randomize the dataset and reduce the selection biases. Univariate and multivariate cox proportional hazards models were utilized to estimate the necessity of chemotherapy. In univariate analysis of matched population, both the overall survival and cancer‐specific survival analysis showed that chemotherapy had no effect on the prognosis of early‐staged young ovarian cancer patients (Overall survival, P = 0.477; Cancer‐specific survival, P = 0.950). In propensity‐adjusted multivariate analysis, chemotherapy still had no effect on both the overall and cancer‐specific survival probability after excluding the effect of all the confounding factors (HR = 0.863, CI = 0.587‐1.269, P = 0.455; HR = 1.009, CI = 0.633‐1.607, P = 0.970). Our study suggested that postoperative chemotherapy is not necessary for early‐staged young ovarian cancer patients with unilateral resection, as indicated by both the overall survival and cancer‐specific survival.
Keywords: chemotherapy, ovarian cancer, premenopausal women, propensity score matching
1. INTRODUCTION
Ovarian cancer, despite of the advances in detection and treatment, is still one of the most malignant tumors in female reproductive system with the highest mortality of 44.6% and the second highest morbidity of 20.9% according to the recent data.1. The peak age of the onset of ovarian cancer is over 50 years old, whereas the premenopausal patients under 50 years account for 38.5% of the whole. It is not well defined that whether chemotherapy should be used for early‐staged ovarian cancer patients undergoing unilateral resection in the guide NCCN 2016. The 5‐year overall and cancer‐specific survival rate were both over 90% based on previous studies. Postoperative chemotherapy has great impact on the quality of life and fertility, especially the young patients who have baby needs. Therefore, it is important to avoid unnecessary chemotherapy. Up to now, whether to undergo postoperative chemotherapy for early‐staged ovarian cancer patients is mainly determined by physician’s experience, which may lead to selection bias.
This study used propensity score matching to analyze the effect of postoperative chemotherapy for early‐staged ovarian cancer patients undergoing unilateral resection on survival rate, which can provide a reference for physicians to reduce the possibility of selection bias.
2. MATERIAL AND METHODS
2.1. Clinical dataset
We included young patients (<50 years old) with early‐staged ovarian cancers diagnosed between 2000 and 2014 from 18 population‐based registries of the Surveillance, Epidemiology, and End Results (SEER), the largest cancer database in the United States, in our study. The SEER database was downloaded from the official website (https://seer.cancer.gov/about/overview.html). Patients without available surgical method or chemotherapy information were excluded from our analysis. Primary ovarian cancer was identified with code "C56.9" according to the International classification of Diseases for Oncology, Third Edition (ICD‐O‐3), and unilateral resection of ovarian was selected by SEER surgical code manual. Chemotherapy information was retrieved individually after getting approval from the SEER official. Besides, we also considered age at diagnosis, marital status, race, tumor grade, American Joint Committee on Cancer (AJCC) stages, tumor size, registry, lateral of original tumor, and histological type for each patient. Cases with no complete survival information including vital status, cause of death, and survival time were removed from further study. We grouped patients into <30 years old, 30‐40 years, and more than 40 years old. Race was classified into four groups of American Indian/Alaska Native (AI/AN), Asian, Black, and White. Tumor grade was classified into well differentiated (G1), moderately differentiated (G2), poorly differentiated (G3), and undifferentiated (G4). We only considered patients with IA, IB, IC, or IIA ovarian cancers based on the criteria of American Joint Committee on Cancer (AJCC) Staging Manual, 7th edition, 2010. Tumor size was divided into three categories by cutoffs of 2 and 10 cm. The 18 registries were grouped into three classes according to the geographical location, central (Metropolitan Detroit, Iowa, Kentucky, Utah, and Louisiana), east (New Jersey, Metropolitan Atlanta, Rural Georgia, and Greater Georgia), and west (Alaska, Greater California, Hawaii, Los Angeles, New Mexico, San Francisco‐Oakland SMSA, San Jose‐Monterey, and Seattle). All the patients were grouped into three histological types epithelial, germ‐cell tumor, and sex‐cord‐stromal tumor according to the ICD‐O‐3 SEER site‐specific manual, and the final analytic set consisted of 1849 cases of patients thereafter.
2.2. Propensity score matching
Propensity score matching was used in the study to avoid the influence of selection bias to the conclusion. Selection bias was generally existed in retrospective studies because of the heterogeneity of demographic and clinical characteristics between the treatment group and control group. A multivariate logistic model was fit by clinical factors including age at diagnosis, marital status, race, tumor grade, tumor stage, tumor size, registry, lateral and tumor histology to predict the probability of a patient receiving chemotherapy. The propensity score was ranged from 0 to 1, and patients with similar propensity scores from the treatment group and control group were matched until all patients in the group with smaller size patients got a match. The nearest neighbor algorithm and one by one match approach were applied in the model, and R package "MatchIT" was used for this analysis.
2.3. Survival analysis
The survival curves were generated by Kaplan‐Meier in the study, and log‐rank test was applied to calculate differences between the curves. Univariate and multivariate cox proportional hazards models were applied for estimating hazard ratios (HRs) and 95% confidence intervals (CI) for each variate by the R package “survival”.
2.4. Statistical analysis
All the statistical analyses in this work were conducted with R version 3.3.2 (https://www.R-project.org/). The differences of clinicopathological characteristics with or without chemotherapy were analyzed by chi‐square (χ 2) test both before and after the propensity score matching. All tests conducted were two‐sided, and significant difference was considered as P‐value <0.05.
3. RESULTS
3.1. Clinical characteristics of the study cohort
We included 1849 early‐staged young ovarian patients who underwent unilateral resection surgery in the study, and 71.9% of the cohort also had no chemotherapy in addition to the surgery. Among the whole population, nearly 50% of the cases were diagnosed at age <30%, and 60.6% of the cohort were single women. White patients constituted 74.2% of the cohort, while the remaining were composed of Asian, Black, and American Indians/Alaska native. Only 52% of patients were available with information of tumor grade, and most of them were well or moderately differentiated (18.8% and 16.8%, respectively). Stage IA and IC patients constituted almost the whole set, with relative 77.5% and 20.4% of the population. For the patients with known tumor sizes, 38.9% of patients had tumors with diameter more than 10 cm. More than half of the study cohort were from western registries, and the primary tumor was equally located on the left or right side of the ovarian. Epithelial ovarian cancer was the most popular in the dataset with the proportion of 55.9%, and sex‐cord‐stromal tumor only made up for 9.1% (Table 1).
Table 1.
Demographic and clinical characteristics of patients with ovarian cancer
| Characteristics | Number | Percentage (%) |
|---|---|---|
| Chemotherapy (%) | ||
| Chemotherapy− | 1330 | 71.9 |
| Chemotherapy+ | 519 | 28.1 |
| Age (%) | ||
| <30 y | 908 | 49.1 |
| 30‐40 y | 450 | 24.3 |
| >40 y | 491 | 26.6 |
| Marital status (%) | ||
| Married | 650 | 35.2 |
| Single | 1121 | 60.6 |
| Unknown | 78 | 4.2 |
| Race (%) | ||
| AI/AN | 13 | 0.7 |
| Asian | 229 | 12.4 |
| Black | 209 | 11.3 |
| White | 1372 | 74.2 |
| Unknown | 26 | 1.4 |
| Grade (%) | ||
| Well differentiated | 347 | 18.8 |
| Moderately differentiated | 310 | 16.8 |
| Poorly differentiated | 223 | 12.1 |
| Undifferentiated | 81 | 4.4 |
| Unknown | 888 | 48 |
| Stage (%) | ||
| IA | 1433 | 77.5 |
| IB | 7 | 0.4 |
| IC | 378 | 20.4 |
| IIA | 31 | 1.7 |
| Tumor Size (%) | ||
| <2 cm | 142 | 7.7 |
| 2‐10 cm | 445 | 24.1 |
| >10 cm | 719 | 38.9 |
| Unknown | 543 | 29.4 |
| Registry (%) | ||
| Central | 328 | 17.7 |
| East | 466 | 25.2 |
| West | 1055 | 57.1 |
| Lateral (%) | ||
| Left | 940 | 50.8 |
| Right | 889 | 48.1 |
| Unknown | 20 | 1.1 |
| Histology (%) | ||
| Epithelial | 1033 | 55.9 |
| Germ‐cell tumor | 647 | 35 |
| Sex‐cord‐stromal tumor | 169 | 9.1 |
3.2. Comparison of covariates before and after propensity score matching
Before the propensity score matching, patients undergoing chemotherapy tended to be diagnosed at younger ages (56.5% vs 46.2% with age <30 years, P < 0.001) and unmarried (32.8% vs 36.1%, P = 0.016). They were less differentiated (7.7% vs 23.1% with well‐differentiated tumor, P < 0.001), less likely to be in stage IA (59.9% vs 84.4%), larger tumor sizes (51.8% vs 33.8% with tumor size more than 10 cm, P < 0.001), and larger proportion of germ‐cell tumors (50.9% vs 28.8%, P < 0.001). Propensity score matching was then performed on the initial dataset to eliminate the heterogeneity and imbalance between the group with or without chemotherapy by building a regression model integrating age at diagnosis, marital status, tumor grade, tumor stage, tumor size and histology. Actually, after the propensity score matching, the two groups with or without chemotherapy were equal in the number of patients, and the clinical factors were well balanced without significant differences, indicating the potential covariates between groups were greatly decreased (Table 2).
Table 2.
Clinical characteristics of the study cohort before and after propensity score matching
| Characteristics | Before Propensity score matching | After Propensity score matching | ||||
|---|---|---|---|---|---|---|
| Chemotherapy− (n = 1330) | Chemotherapy+ (n = 519) | P‐value | Chemotherapy− (n = 1330) | Chemotherapy+ (n = 519) | P‐value | |
| Age (%) | ||||||
| <30 y | 615 (46.2) | 293 (56.5) | <0.001 | 307 (59.2) | 293 (56.5) | 0.588 |
| 30‐40 y | 344 (25.9) | 106 (20.4) | 94 (18.1) | 106 (20.4) | ||
| >40 y | 371 (27.9) | 120 (23.1) | 118 (22.7) | 120 (23.1) | ||
| Marital status (%) | ||||||
| Married | 480 (36.1) | 170 (32.8) | 0.016 | 154 (29.7) | 170 (32.8) | 0.561 |
| Single | 785 (59.0) | 336 (64.7) | 351 (67.6) | 336 (64.7) | ||
| Unknown | 65 (4.9) | 13 (2.5) | 14 (2.7) | 13 (2.5) | ||
| Race (%) | ||||||
| American Indian | 8 (0.6) | 5 (1.0) | 0.36 | 2 (0.4) | 5 (1.0) | 0.634 |
| Asian | 160 (12.0) | 69 (13.3) | 70 (13.5) | 69 (13.3) | ||
| Black | 142 (10.7) | 67 (12.9) | 59 (11.4) | 67 (12.9) | ||
| White | 999 (75.1) | 373 (71.9) | 380 (73.2) | 373 (71.9) | ||
| Unknown | 21 (1.6) | 5 (1.0) | 8 (1.5) | 5 (1.0) | ||
| Grade (%) | ||||||
| Well differentiated | 307 (23.1) | 40 (7.7) | <0.001 | 44 (8.5) | 40 (7.7) | 0.071 |
| Moderately differentiated | 195 (14.7) | 115 (22.2) | 131 (25.2) | 115 (22.2) | ||
| Poorly differentiated | 105 (7.9) | 118 (22.7) | 98 (18.9) | 118 (22.7) | ||
| Undifferentiated | 33 (2.5) | 48 (9.2) | 29 (5.6) | 48 (9.2) | ||
| Unknown | 690 (51.9) | 198 (38.2) | 217 (41.8) | 198 (38.2) | ||
| Stage (%) | ||||||
| IA | 1122 (84.4) | 311 (59.9) | <0.001 | 353 (68.0) | 311 (59.9) | 0.045 |
| IB | 6 (0.5) | 1 (0.2) | 1 (0.2) | 1 (0.2) | ||
| IC | 183 (13.8) | 195 (37.6) | 152 (29.3) | 195 (37.6) | ||
| IIA | 19 (1.4) | 12 (2.3) | 13 (2.5) | 12 (2.3) | ||
| Tumor size (%) | ||||||
| <2 cm | 127 (9.5) | 15 (2.9) | <0.001 | 11 (2.1) | 15 (2.9) | 0.601 |
| 2‐10 cm | 318 (23.9) | 127 (24.5) | 119 (22.9) | 127 (24.5) | ||
| >10 cm | 450 (33.8) | 269 (51.8) | 266 (51.3) | 269 (51.8) | ||
| Unknown | 435 (32.7) | 108 (20.8) | 123 (23.7) | 108 (20.8) | ||
| Registry (%) | ||||||
| Central | 225 (16.9) | 103 (19.8) | 0.12 | 82 (15.8) | 103 (19.8) | 0.191 |
| East | 327 (24.6) | 139 (26.8) | 137 (26.4) | 139 (26.8) | ||
| West | 778 (58.5) | 277 (53.4) | 300 (57.8) | 277 (53.4) | ||
| Lateral (%) | ||||||
| Left | 682 (51.3) | 258 (49.7) | 0.776 | 280 (53.9) | 258 (49.7) | 0.385 |
| Right | 633 (47.6) | 256 (49.3) | 235 (45.3) | 256 (49.3) | ||
| Unknown | 15 (1.1) | 5 (1.0) | 4 (0.8) | 5 (1.0) | ||
| Histology (%) | ||||||
| Epithelial | 811 (61.0) | 222 (42.8) | <0.001 | 221 (42.6) | 222 (42.8) | 0.479 |
| Germ‐cell tumor | 383 (28.8) | 264 (50.9) | 255 (49.1) | 264 (50.9) | ||
| Sex‐cord‐stromal tumor | 136 (10.2) | 33 (6.4) | 43 (8.3) | 33 (6.4) | ||
3.3. Chemotherapy has no significant benefit on the survival of early‐staged ovarian cancer patients undergoing unilateral resection
Chemotherapy demonstrated no significant benefit to patients with early‐staged ovarian cancer who underwent unilateral resection for both overall survival and cancer‐specific survival (Overall survival, P = 0.396; Cancer‐specific survival, P = 0.996; Figure 1). For all the stages included in this study, patients with chemotherapy did not show any survival differences compared with those underwent no chemotherapy (Table 3 and Supplementary Figure S1).
Figure 1.
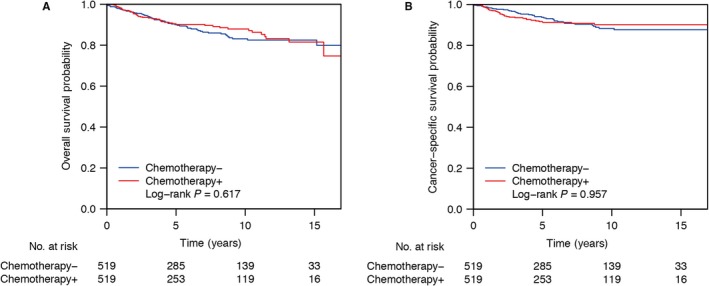
Chemotherapy demonstrated no significant benefit to patients with early‐staged ovarian cancer who underwent unilateral resection for both overall survival and cancer‐specific survival. Overall survival probability (A) or cancer‐specific survival probability (B) of patients with early‐staged ovarian cancer who underwent unilateral resection underwent or without chemotherapy
Table 3.
Five year survival probability in different stages of ovarian cancer
| Overall survival | Cancer‐specific survival | |||||
|---|---|---|---|---|---|---|
| Chemotherapy− | Chemotherapy+ | P‐value | Chemotherapy− | Chemotherapy+ | P‐value | |
| IA | 0.918 (0.017) | 0.933 (0.016) | 0.396 | 0.947 (0.014) | 0.941 (0.015) | 0.996 |
| IB | 1 (0) | 1 (0) | 1 | 1 (0) | 1 (0) | 1 |
| IC | 0.861 (0.033) | 0.854 (0.03) | 0.864 | 0.905 (0.029) | 0.871 (0.029) | 0.902 |
| IIA | 0.666 (0.138) | 0.771 (0.144) | 0.491 | 0.666 (0.138) | 0.771 (0.144) | 0.491 |
3.4. Univariate and multivariate analysis
We conducted univariate cox proportional hazard analyses on the matched population of all the clinical characteristics to explore their prognostic effect (Table 4). Both the overall survival and cancer‐specific survival analysis showed that chemotherapy had no effect on the prognosis of early‐staged young ovarian cancer patients (Overall survival, P = 0.477; Cancer‐specific survival, P = 0.950). Older ages demonstrated a hazard factor for both the overall and cancer‐specific survival (P < 0.001), while race only showed overall survival differences among groups (P = 0.007). Both tumor grade and stage were significantly associated with overall and cancer‐specific survival (P < 0.001), and tumor size was only correlated with cancer‐specific survival probability (P = 0.042). Besides, histology demonstrated a significant risk factor for both the overall and survival rate (P < 0.001). Further, we took all the risk factors associated with survival from the univariate analysis into a multivariate cox proportional model with chemotherapy, and the result was shown in Table 5. Age more than 40 years remained a risk factor in the overall survival but not for the cancer‐specific survival (HR = 2.372, CI = 1.329‐4.233, P = 0.003; HR = 1.261, CI = 0.672‐2.365, P = 0.471). Stage IC was associated with poorer survival probabilities than stage IA patients (HR = 1.753, CI = 1.178‐2.609, P = 0.006; HR = 2.212, CI = 1.357‐3.604, P = 0.001), while stage IIA only correlated with worse cancer‐specific survival (HR = 3.502, CI = 1.426‐8.599, P = 0.006). Poorly differentiated (HR = 5.801, CI = 1.769‐19.010, P = 0.004; HR = 5.089, CI = 1.520‐17.030, P = 0.008) and undifferentiated tumors (HR = 5.768, CI = 1.604‐20.730, P = 0.007; HR = 5.107, CI = 1.356‐19.230, P = 0.016) were both associated with worse survival compared to the well‐differentiated tumor. Chemotherapy still had no effect on both the overall and cancer‐specific survival probability after excluding the effect of all the confounding factors (HR = 0.863, CI = 0.587‐1.269, P = 0.455; HR = 1.009, CI = 0.633‐1.607, P = 0.970).
Table 4.
Univariate analysis of the matched population for overall and cancer‐specific survival
| Characteristics | N | Overall | Cancer‐specific | ||
|---|---|---|---|---|---|
| 5‐year survival (%) | P‐value | 5‐year survival (%) | P‐value | ||
| Chemotherapy (%) | |||||
| Chemotherapy− | 519 | 0.895 | 0.477 | 0.928 | 0.950 |
| Chemotherapy+ | 519 | 0.901 | 0.912 | ||
| Age (%) | |||||
| <30 y | 600 | 0.960 | <0.001 | 0.967 | <0.001 |
| 30‐40 y | 200 | 0.907 | 0.922 | ||
| >40 y | 238 | 0.752 | 0.811 | ||
| Marital Status (%) | |||||
| Married | 324 | 0.892 | 0.835 | 0.903 | 0.612 |
| Single | 687 | 0.902 | 0.929 | ||
| Unknown | 27 | 0.882 | 0.941 | ||
| Race (%) | |||||
| American Indian | 7 | 1.000 | 0.007 | 1.000 | 0.106 |
| Asian | 139 | 0.966 | 0.966 | ||
| Black | 126 | 0.899 | 0.912 | ||
| White | 13 | 1.000 | 1.000 | ||
| Unknown | 753 | 0.885 | 0.912 | ||
| Grade (%) | |||||
| Well differentiated | 84 | 0.953 | <0.001 | 0.953 | <0.001 |
| Moderately differentiated | 246 | 0.906 | 0.932 | ||
| Poorly differentiated | 216 | 0.820 | 0.859 | ||
| Undifferentiated | 77 | 0.840 | 0.851 | ||
| Unknown | 415 | 0.935 | 0.951 | ||
| Stage (%) | |||||
| IA | 664 | 0.925 | <0.001 | 0.944 | <0.001 |
| IB | 2 | 1.000 | 1.000 | ||
| IC | 347 | 0.856 | 0.885 | ||
| IIA | 25 | 0.718 | 0.718 | ||
| Tumor Size (%) | |||||
| <2 cm | 26 | 0.800 | 0.067 | 0.800 | 0.042 |
| 2‐10 cm | 246 | 0.874 | 0.896 | ||
| >10 cm | 535 | 0.918 | 0.937 | ||
| Unknown | 231 | 0.889 | 0.920 | ||
| Registry (%) | |||||
| Central | 185 | 0.881 | 0.708 | 0.919 | 0.976 |
| East | 276 | 0.895 | 0.917 | ||
| West | 577 | 0.905 | 0.922 | ||
| Lateral (%) | |||||
| Left | 538 | 0.903 | 0.188 | 0.929 | 0.048 |
| Right | 491 | 0.899 | 0.918 | ||
| Unknown | 9 | 0.571 | 0.571 | ||
| Histology (%) | |||||
| Epithelial | 443 | 0.808 | <0.001 | 0.840 | <0.001 |
| Germ‐cell tumor | 519 | 0.987 | 0.998 | ||
| Sex‐cord‐stromal tumor | 76 | 0.833 | 0.865 | ||
Table 5.
Multivariate cox proportional model of the matched population for overall and cancer‐specific survival
| Overall | Cancer‐specific | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age (%) | ||||||
| <30 y | Ref | Ref | ||||
| 30‐40 y | 1.193 | 0.613‐2.319 | 0.603 | 1.074 | 0.5252.197 | 0.846 |
| >40 y | 2.372 | 1.329‐4.233 | 0.003 | 1.261 | 0.672‐2.365 | 0.471 |
| Race (%) | ||||||
| American Indian | Ref | Ref | ||||
| Asian | 2813953.873 | 0‐Inf | 0.995 | 4505754.487 | 0‐Inf | 0.996 |
| Black | 7987742.825 | 0‐Inf | 0.995 | 12587762.630 | 0‐Inf | 0.996 |
| White | 6835996.312 | 0‐Inf | 0.995 | 7511476.490 | 0‐Inf | 0.996 |
| Unknown | 1.139 | 0‐Inf | 1.000 | 1.440 | 0‐Inf | 1.000 |
| Stage (%) | ||||||
| IA | Ref | Ref | ||||
| IB | 0.000 | 0‐Inf | 0.999 | 0.000 | 0‐Inf | 0.999 |
| IC | 1.753 | 1.178‐2.609 | 0.006 | 2.212 | 1.357‐3.604 | 0.001 |
| IIA | 1.926 | 0.812‐4.562 | 0.136 | 3.502 | 1.426‐8.599 | 0.006 |
| Grade (%) | ||||||
| Well differentiated | Ref | Ref | ||||
| Moderately differentiated | 3.092 | 0.925‐10.33 | 0.067 | 2.287 | 0.662‐7.903 | 0.191 |
| Poorly differentiated | 5.801 | 1.769‐19.01 | 0.004 | 5.089 | 1.520‐17.03 | 0.008 |
| Undifferentiated | 5.768 | 1.604‐20.73 | 0.007 | 5.107 | 1.356‐19.23 | 0.016 |
| Unknown | 3.311 | 0.997‐10.99 | 0.051 | 2.277 | 0.655‐7.917 | 0.196 |
| Histology (%) | ||||||
| Epithelial | Ref | Ref | ||||
| Germ‐cell Tumor | 0.161 | 0.072‐0.360 | <0.001 | 0.017 | 0.002‐0.126 | <0.001 |
| Sex‐cord‐stromal Tumor | 0.910 | 0.455‐1.818 | 0.790 | 0.736 | 0.333‐1.623 | 0.447 |
| Chemotherapy (%) | ||||||
| Chemotherapy− | Ref | Ref | ||||
| Chemotherapy+ | 0.863 | 0.587‐1.269 | 0.455 | 1.009 | 0.633‐1.607 | 0.970 |
4. DISCUSSION
We conducted a population‐based study on young women with early‐staged ovarian cancer to explore the necessity of postoperative chemotherapy for such patients who undergo unilateral resection. The propensity score matching was used to help randomize the dataset and strengthen causal arguments by reducing selection bias of diagnosis. The univariate and multivariate cox proportional hazard model analysis suggested that postoperative chemotherapy is not necessary for young patients undergoing unilateral resection therapy.
For young women of childbearing age, the reproductive ability has to be taken into account. Combination therapy of postoperative patients with cisplatin and paclitaxel is recommended by the NCCN guide.2 Cisplatin directly binds to the DNA of tumor cells, forming a cross‐link that leads to the arrest of DNA synthesis and replication, resulting in apoptosis.3 Paclitaxel mainly blocks cancer growth by binding to the tubulin proteins needed for cell division.4 However, both of the drugs may cause several types of side effects due to the lack of selectivity and the cytotoxicity of the target. One of the major side effects that cannot be neglected is the reproductive toxicity due to the disease’s sex selection. For pregnant women, chemotherapy with cisplatin and paclitaxel during the second and third trimesters with these two drugs may lead to a relatively high risk of premature rupture of membranes (PROM), intrauterine growth restriction (IUGR), and premature labor,5, 6 according to available data. Besides, first trimester chemotherapy exposure is associated with fetal malformations, spontaneous abortions, and fetal death.7 In clinical practice, women undergoing chemotherapy should avoid pregnancy, and termination should be considered in patients with cancer who need systemic treatment in the first trimester. Therefore, the exemption of postoperative chemotherapy means a lot to the early‐staged young women with ovarian preservation and willing to have a child.
Aside from the concerns for reproductive ability, there are some other side effects in the use of cisplatin and paclitaxel, such as bone marrow suppression, which may lead to anemia, infection, and fever.8 Neurotoxicity, which damages motor and sensory nerves.9, 10 Nausea, vomiting, and dermatitis are also common symptoms after using these two drugs.11 In addition, the nephrotoxicity of cisplatin may lead to acute kidney injury (AKI) or irreversible renal dysfunction.12 Hence, the conduction of postoperative chemotherapy should be cautiously considered by the operatives.
Even though we included large number of cases and applied bias reduction method to ensure the accuracy and reliability of our study, there are still some concerns that need to be pointed out. Firstly, we didn’t consider the individual difference and assuming women younger than 50 years old were premenopausal. Secondly, as the information of specific drugs in chemotherapy was not available in the database, we only considered the influence of whether applying the therapy or not. Inclusion of chemotherapy dose and duration is preferred in further analysis to give more precise conclusion. Besides, despite that we considered as many as potential clinical cofactors in our analysis, the limited information on surgical and treatment options such as the procedure strategy, specimen adequacy, and the judgement of pathologists was still overlooked for their influence on prognosis.
In summary, our study suggested that postoperative chemotherapy is not necessary for early‐staged young ovarian cancer patients with unilateral resection, as indicated by both the overall survival and cancer‐specific survival. The exemption of postoperative chemotherapy will do great benefit to young women with childbearing ability and wishes without reducing the curative effects. Nevertheless, a comprehensive risk assessment from the physicians and associated tests is strongly recommended. In addition, randomized clinical trials are needed to further evaluate the necessity of postoperative chemotherapy for targeted patients.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
Supporting information
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (nos.81572553 and 81372797 for G. Yang).
Zhang X, Wang S, Zhao S, Sun Y, Yang G. Postoperative chemotherapy had no prognostic effect on early‐staged young ovarian cancer with unilateral resection. Cancer Med. 2018;7:5488–5496. 10.1002/cam4.1822
Xiaofei Zhang and Shuoer Wang contributed equally to this work.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Morgan RJ, Armstrong DK, Alvarez RD, et al. Ovarian cancer, version 1.2016, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN. 2016;14(9):1134‐1163. [DOI] [PubMed] [Google Scholar]
- 3. Sedletska Y, Giraud‐panis MJ, Malinge JM. Cisplatin is a DNA‐damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Curr Med Chem Anticancer Agents. 2005;5(3):251‐265. [DOI] [PubMed] [Google Scholar]
- 4. Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677‐2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boussios S, Moschetta M, Tatsi K, Tsiouris AK, Pavlidis N. A review on pregnancy complicated by ovarian epithelial and non‐epithelial malignant tumors: diagnostic and therapeutic perspectives. J Adv Res. 2018;12:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serkies K, Wegrzynowicz E, Jassem J. Paclitaxel and cisplatin chemotherapy for ovarian cancer during pregnancy: case report and review of the literature. Arch Gynecol Obstet. 2011;283(Suppl 1):97‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leslie KK, Koil C, Rayburn WF. Chemotherapeutic drugs in pregnancy. Obstet Gynecol Clin North Am. 2005;32(4):627‐640. [DOI] [PubMed] [Google Scholar]
- 8. Hara T, Nishikawa K, Sakatoku M, Oba K, Sakamoto J, Omura K. Phase II study of weekly paclitaxel, cisplatin, and 5‐fluorouracil for advanced gastric cancer. Gastric Cancer. 2011;14(4):332‐338. [DOI] [PubMed] [Google Scholar]
- 9. Gornstein E, Schwarz TL. The paradox of paclitaxel neurotoxicity: Mechanisms and unanswered questions. Neuropharmacology. 2014;76(Pt A):175‐183. [DOI] [PubMed] [Google Scholar]
- 10. Boehmerle W, Huehnchen P, Peruzzaro S, Balkaya M, Endres M. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib‐induced neuropathy in C57Bl/6 mice. Sci Rep. 2014;4:6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Donati A, Castro LG. Cutaneous adverse reactions to chemotherapy with taxanes: the dermatologist's point of view. An Bras Dermatol. 2011;86(4):755‐758. [DOI] [PubMed] [Google Scholar]
- 12. Ozkok A, Edelstein CL. Pathophysiology of cisplatin‐induced acute kidney injury. BioMed Res Int. 2014;2014:967826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


