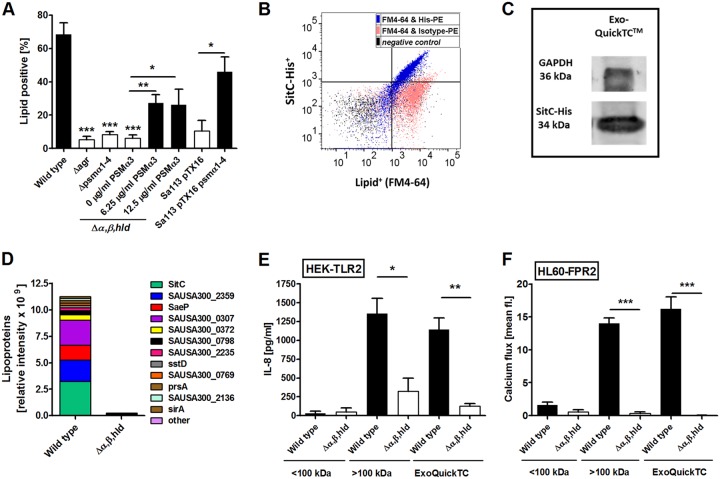
FIG 3.
Composition and host-activating capacity of MVs isolated from wild-type and Δα,β,hld bacterial cultures. (A) Flow cytometry analysis of MVs recovered from strains without PSM expression such as USA300 Δagr, USA300 Δpsmα1-4, the PSM mutant (Δα,β,hld; lacking all PSMs), or the agr-deficient laboratory strain SA113 revealed substantially reduced MV amounts. Addition of synthetic PSMα3 to the PSM mutant culture, or complementation of SA113 with a plasmid carrying psmα1-4, successfully restored the release of MVs. (B) Lipid membrane (FM4-64+)-positive particles from USA300 Δspa pTX SitC-His are also SitC-His positive when analyzed by flow cytometry. (C) Immunoblotting of wild-type ExoQuickTC-isolated MVs detecting the cytoplasmic protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the model lipoprotein SitC. (D) Proteomic analysis reveals the presence of other lipoproteins in addition to SitC in wild-type ExoQuickTC-isolated MVs. (E) Larger-than-100-kDa and MV fractions from wild type can activate TLR2-transfected HEK293 cells, resulting in the secretion of IL-8 cytokines. All <100-kDa fractions as well as all Δα,β,hld fractions fail to induce a strong IL-8 secretion. (F) Wild-type MV and high-molecular-weight (>100-kDa) fractions induce calcium influx in FPR2-transfected HL60 cells. All other fractions fail to induce calcium influx. Data in panels A, E, and F represent means ± SEM from at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001, significant difference versus USA300 wild type, as calculated by the unpaired (A) or paired (E and F) two-tailed Student t test. Data in panel D represent means of three independent experiments, and data in panels B and C are each representative of three independent experiments.