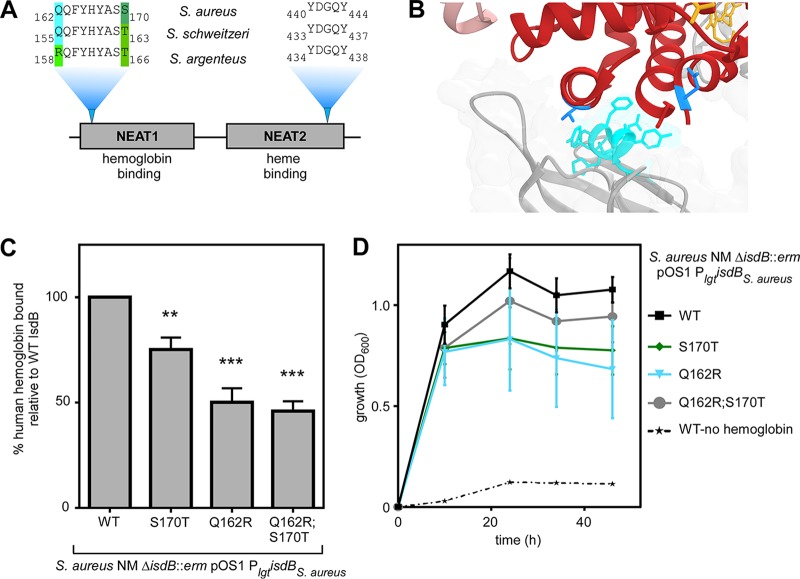
FIG 6.
IsdB NEAT1 domain diversity among staphylococci modulates human hemoglobin recognition. (A) An alignment of the NEAT1 subdomain critical for hemoglobin binding shows variation among staphylococcal IsdB, while no variation was observed for the NEAT2 subdomain required for heme binding. (B) The Q162 to S170 subdomain of NEAT1 (cyan) is proximal to helices containing T8 and N78 of α-globin (red). (C) S. aureus lacking native isdB but harboring constitutively expressed plasmid-borne S. aureus isdB variants was incubated with purified recombinant human hemoglobin, and bound hemoglobin was quantified. (D) The growth of S. aureus lacking native isdB but harboring constitutively expressed plasmid-borne S. aureus isdB variants using hemoglobin as the sole iron source was monitored over time. Panel C shows, the means from three independent experiments with 3 biological replicates ± SEM; **, P < 0.005; ***, P < 0.0005 by two-way ANOVA with Sidak’s correction for multiple comparisons, comparing transformed (percent value) data. Panel D shows the results of two independent experiments with six biological replicates each ± standard deviations.