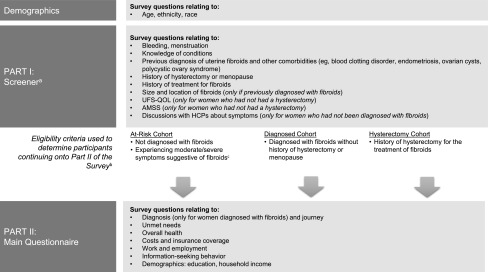
FIG. 1.
Overview of Survey and Questions. aScreening questions also included consent to participate and determined qualification to participate in the remainder of the study. bParticipants who did not meet eligibility criteria did not see questions included in Part II of the survey. cBased on responses to the UFS-QOL and select questions from the AMSS portion of the Screener (Part I), severe or moderate uterine fibroids were defined as at least a moderate or higher score on both transformed UFS-QOL measures (Symptom Severity ≥36.5 and HRQOL Total Score ≤72.5) and the AMSS (≥40.5) or a severe score on at least one of the following metrics: UFS-QOL Symptom Severity ≥56.5, UFS-QOL HRQOL Total Score ≤50.5, or AMSS ≥72.5. AMSS, Aberdeen Menorrhagia Severity Scale; HCP, healthcare provider; UFS-QOL, Uterine Fibroid Symptom and Health-Related Quality of Life.