Abstract
In 1958, Neil Wald presented data on the incidence of leukemia among the Hiroshima atomic bomb survivors. These data, which suggested a dose–response threshold for radiation-induced leukemia, were included in the first UNSCEAR report (1958). However, this evidence of a threshold was not recognized. It was obfuscated and concealed. In 2010, Zbigniew Jaworowski identified these data as evidence of radiation hormesis. A letter to the editor in 2014 and 2 articles in 2014 and 2015 presented a graph of these UNSCEAR 1958 data, which revealed a threshold at about 500 mSv. Since the blood-forming stem cells of bone marrow are more radiosensitive than most other cell types, it is reasonable to expect thresholds for inducing other types of cancer by ionizing radiation—their thresholds are likely higher than 500 mSv. A careful examination of the Wald data reveals the suprisingly low incidence of radiogenic leukemia, only 0.5% of the survivors who were in the high radiation zone. Many articles on radiation risk have been published since 2015 by other authors, but none makes reference to this evidence of a threshold, either to challenge or endorse it. In this commentary, the author addresses the comments from a colleague.
Keywords: ionizing radiation, Hiroshima atomic bomb survivors, dose–response threshold, leukemia, cancer, hormesis
Introduction
Widespread fear of low-dose ionizing radiation (LDIR) began in 1956 when the US National Academy of Sciences (NAS) recommended that the linear no-threshold (LNT) dose–response model be used to assess the risk of radiation-induced mutations.1 Nuclear power plants and all applications of LDIR, especially in medicine, began to be linked to a risk of dreaded cancer. Prior to this NAS publication and the associated publicity, there had been 60 years of extensive experience using X-rays and radium to image and treat many millions of patients. The dose rate limit (tolerance dose) for protecting radiologists against overexposures was based on a threshold model, and it was satisfactory.2,3 There were no reports of elevated cancer levels, when the early radiation protection standards were followed. On the contrary, lower cancer mortality and increased longevity were observed in follow-up studies of radiologists and nuclear workers.4,5
In addition to the diagnostic applications, many treatments with LDIR were discovered and employed on many millions of adults and children against very serious diseases, including a variety of cancers, infections, and inflammations.2 Low radiation doses were observed to be stimulatory (beneficial). A National Cancer Institute review of nasopharyngeal radium irradiation (NRI) reported that worldwide studies have not confirmed a definite link between NRI and any disease.6
It was recently discovered that the 1956 NAS recommendation was ideologically motivated and was based on the deliberate falsification and fabrication of the research record. This NAS scientific misconduct led to governments adopting the LNT model for cancer risk assessment.7-9 Many scientists wanted to stop the ongoing development of nuclear weapons after 2 atomic bombs were used to end World War II. Radiophobia was promoted as part of a political strategy to stop all atomic bomb testing, which releases radioactive materials (fallout) into the environment. More than 60 years have passed since that NAS recommendation, but the fear of radiation is sustained by regulatory disregard of the large amount of evidence that contradicts it.10
This commentary reviews the UNSCEAR 1958 data and endeavors to understand why this evidence of a threshold for radiation-induced leukemia is being ignored by other authors, even those who have been challenging the validity of the LNT model of radiation carcinogenesis. They do not make reference to this UNSCEAR information, either to challenge or endorse it. In this commentary, the author addresses the comments from a colleague.
Incidence of Leukemia in the Hiroshima Survivors
In 1958, Niel Wald summarized the results of the leukemia survey in Hiroshima as of December 1957. The numbers of cases for the years 1950 through 1956 are fairly accurate; however, the numbers that arose in the preceding years are significantly understated. With respect to 1957, there were likely additional cases discovered.11 Table 1 is the original table of this information and Figure 1 is a graph of the number of cases versus year.
Table 1.
Leukemia in Hiroshima Atomic Bomb Survivors Who Were Residents of Hiroshima City at the Time of Diagnosis, as of December 1957.11
| Year of Onset | Total | Distance From Hypocenter (meters) | ||||
|---|---|---|---|---|---|---|
| Under 1000 | 1000-1499 | 1500-1999 | 2000-2999 | 3000 and Over | ||
| 1945 | ||||||
| 1946 | ||||||
| 1947 | 3 | 1 | 2 | |||
| 1948 | 7 | 2 | 4 | 1 | ||
| 1949 | 5 | 1 | 1 | 1 | 1 | 1 |
| 1950 | 9 | 3 | 5 | 1 | ||
| 1951 | 11 | 3 | 7 | 1 | ||
| 1952 | 11 | 3 | 5 | 1 | 2 | |
| 1953 | 12 | 2 | 6 | 2 | 1 | 1 |
| 1954 | 6 | 2 | 2 | 1 | 1 | |
| 1955 | 8 | 1 | 4 | 2 | 1 | |
| 1956 | 6 | 1 | 1 | 1 | 3 | |
| 1957 | 5 | 1 | 3 | 1 | ||
| Total | 83 | 18 | 39 | 9 | 7 | 10 |
| Estimated population* | ||||||
| 95 819 | 1241 | 8810 | 20 113 | 32 692 | 32 963 | |
| Number of cases with onset in 1950-1957 | ||||||
| 68 | 15 | 33 | 8 | 3 | 9 | |
| Estimated person-years at risk | ||||||
| 766 552 | 9928 | 70 480 | 160 904 | 261 536 | 263 704 | |
| Annual incidence of leukemia per 100,000 | ||||||
| 8.9 | 151.1 | 46.8 | 5.0 | 1.1 | 3.4 | |
*Based on Hiroshima Census Bureau’s daytime population census of Hiroshima City, June 3, 1953.
Figure 1.
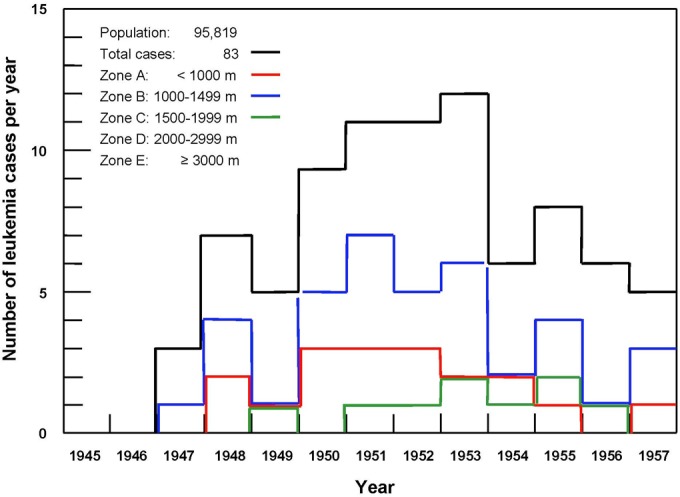
Number of leukemia cases per year.
Wald’s data were included in the first UNSCEAR report (1958), Annex G, Table VII (Table 2 below).12 Zbigniew Jaworowski, representative of Poland in UNSCEAR, referred to these data in an article advocating the use of radiation hormesis as a remedy for fear.10 He stated on page 266, “hormesis is clearly evident…in a table showing leukemia incidence in the Hiroshima population, which was lower by 66.3% in survivors exposed to 20 mSv, compared to the unexposed group (p.165). This evidence of radiation hormesis was not commented upon.”
Table 2.
UNSCEAR 1958. Table VII. Leukemia Incidence 1950-57 After Exposure at Hiroshima.a
| Zone | Distance From Hypocenter (m) | Dose (REM) | Persons Exposed | L (Cases of Leukemia) | Nb (Total Cases per 106) | ||||
|---|---|---|---|---|---|---|---|---|---|
| A | Under 1000 | 1300 | 1241 | 15 | 3.9 | 12 087 ± 3143 | |||
| B | 1000-1499 | 500 | 8810 | 33 | 5.7 | 3746 ± 647 | |||
| C | 1500-1999 | 50c | 20 113 | 8 | 2.8 | 398 ± 139 | |||
| D | 2000-2999 | 2 | 32 692 | 3 | 1.7 | 92 ± 52 | |||
| E | Over 3000 | 0 | 32 963 | 9 | 3.0 | 273 ± 91 | |||
A graph was made of these data, Figure 2, and this evidence of a threshold at about 500 mSv was presented in a letter to Archive of Toxicology and an article in Dose-Response.2,13 A year passed and it became apparent that this very important evidence was being ignored by the scientific community and the media. Another article was prepared in 2015 that criticized a 1957 article by Edward Lewis. This article demonstrated that Lewis had misled the scientific community by combining 2 exposed population groups, averaging their doses and concealing the evidence of the threshold.14 (A threshold would have contradicted the LNT model.) Although this article has been viewed 8810 times on the Internet and referenced by the author in several additional articles, it has not been cited by other authors.
Figure 2.
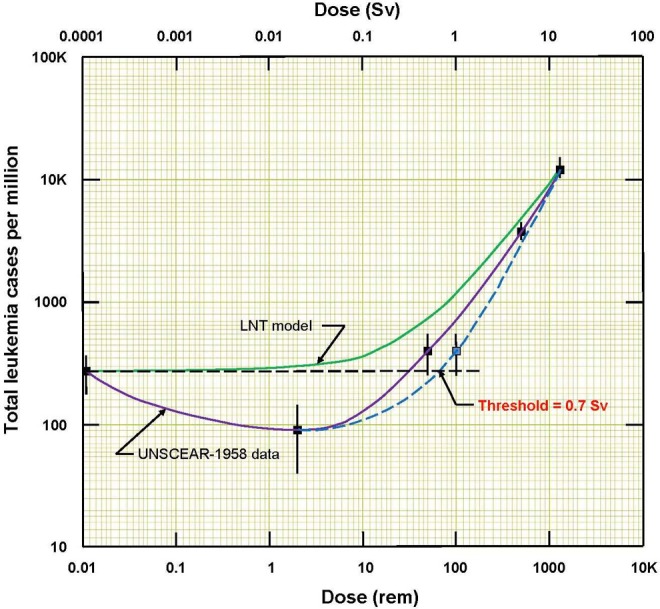
Total number of leukemia cases per million versus radiation dose. Data from UNSCEAR 1958.12 Evidence of a threshold for radiogenic leukemia is apparent at about 0.7 Sv, or 0.7 Gy assuming RBE = 1.
Review of the 2015 Article by a Colleague
A recently published critical evaluation of the NCRP Commentary 27 endorsement of the LNT model15 did not mention the UNSCEAR 1958 evidence of a threshold for radiogenic leukemia that appears in the 2015 article.14 When the author of the evaluation was asked why this important evidence had been omitted, he provided the following comments.
The conclusion that the acute dose threshold for leukemia is 500 mSv is extraordinary. It is in stark contrast to conventional knowledge—the difference being about one or two orders of magnitude.
Skepticism is created by changing the Zone C dose from the calculated value of 0.5 Sv to the value 1 Sv, to address the footnote: “almost all cases of leukemia in this zone occurred in patients who had severe radiation complaints, indicating that their doses were greater than 50 rem.” A more careful reading led to an understanding of the rationale for this change and the acknowledgement that the Zone C dose could have been raised even higher.
The conclusion that the radiation thresholds for other cancer types are expected to be higher than the 500 mSv threshold for excess leukemia is of significant concern. There exists an additional 42 years of follow-up leukemia data that should be discussed. To extend that claim to other types of cancer would require an evaluation of the most recent solid cancer incidence/mortality data, which was not carried out.
There is no discussion of the optimum time window for detecting putative radiation-induced leukemia, which is the first 10 to 15 years following an acute exposure. The idea that including years of data afterward just dilutes the effect merits further discussion. The initial leukemia signal is most visible in that time window and fades toward the null of no effect, as more and more naturally occurring leukemia cases accumulate in both the exposed and control groups with the passage of time. The RERF data updates should have been analyzed.
Responses to the Reviewer’s Comments
Indeed, the reported threshold dose to induce leukemia, about 500 mSv, is 1 or 2 orders of magnitude higher than the currently accepted level of significant risk. Conventional knowledge is based on applying the LNT model, which continues to be discredited. The threshold is a factor of 5 higher than the 100 mSv value that many radiation protection people seem willing to accept. Up until the 1960s, millions of patients received repeated radiation doses in range from about 0.1 to 1 ED (erythema dose ≈ 600 mSv) to cure many life-threatening diseases. There are no reports of a significant increase in leukemia incidence following such treatments.6 Many Chernobyl firefighters suffered from very high radiation doses; 134 of them were treated for acute radiation syndrome. Of them, 28 died within weeks and 106 recovered. Follow-up of these 106 survivors after 19 years showed no increase in their overall mortality or their cancer mortality compared to unexposed workers.3 And there have been other accidents involving exposures of many people to high radiation levels that resulted in serious burns, but no evidence of elevated cancer incidence. Doss has suggested that there is a fundamental weakness in the somatic mutation model of cancer being used. He recommends more attention be given to the immune suppression model of cancer.16 Indeed, it is well known that a high dose of radiation suppresses immunity and increases the risk of cancer.17 Since the acute lethal dose for humans ranges from 3.5 to 5 Gy,18 a threshold for the onset of radiogenic leukemia at about 1 Gy is credible.
Changing the dose for Zone C was very important because a dose that is based on actual human symptoms is much more credible than a dose that is calculated using a primitive model of atomic bomb radiation.
The Hiroshima evidence of radiogenic leukemia can be modeled by a hormetic dose–response model.2,14 Since we know that LNT is wrong, it is likely that the other radiogenic cancer types can be modeled likewise. It is reasonable to expect the threshold doses for other cancer types to be higher than for leukemia because of the discussion in the 2012 article by Fliedner et al on the high radiation sensitivity of hemopoietic stem cells compared with the radiation sensitivities of stem cells in other organs.19
The long-term studies on radiation-induced leukemia mortality and the mortality of other cancers among the bomb survivors lack credibility because the LNT model is invalid. Cancer and the effects of radiation on cancer mortality are not well understood. The confounding factors that affect radiogenic cancer mortality are not known and, if they were, it would be impossible to control them over many decades. There is no value to be gained in analyzing RERF data updates.
An assessment of the 1958 to 2000 bomb survivor leukemia data20 was not included in the 2014 and 2015 articles,2,14 and unfortunately, no explanation was given for this omission. It was known that radiogenic leukemia has a short latent period. The excess cases appear a few years after the irradiation and reach a peak by 5 to 7 years. Most radiogenic leukemia cases occur in the first 15 years. Solid tumors show a longer latency, from 10 to 60 years or more.21 Clearly, the inclusion of the leukemia data from 1958 to 2000 would have diluted the burst of radiogenic leukemia cases with 43 years of naturally occurring leukemia cases, about 3 per 100 000 per year, masking the evidence of the radiogenic leukemia dose threshold.
Table 1 shows the leukemia data of the 95 819 survivors from 1945 until the end of 1957.11 Figure 1 shows that the radiogenic cases began to appear in 1948 and peaked from 1950 until the end of 1953. In the 2014 and 2015 articles,2,14 it was appropriate to do as the UNSCEAR-1958 report12 did—examine the cases in the 8-year interval 1950 to 1957 to evaluate the dependence of radiation-induced leukemia on dose. Figure 2 shows the leukemia incidence response to radiation dose. A threshold for radiogenic leukemia is apparent at an “equivalent” dose of about 0.7 Sv, or 0.7 Gy (70 rad) in “absorbed” dose units, assuming RBE = 1. The 32 963 people who were in the outermost Zone E are regarded as the nonexposed controls. Their annual (natural) leukemia incidence is 3.4 cases per 100 000, as given in Table 1.
The uncertainty of the threshold can be gauged by noting that 0.7 Gy is 30% below the assumed 1 Gy dose for severe radiation pain, the spread of which is likely the same as the human LD50 range, which is 3.5-50 Gy.18
Conclusions
The data on the incidence of leukemia among the Hiroshima atomic bomb survivors, which were summarized by Neil Wald and included in the 1958 UNSCEAR report, are evidence of a dose threshold for radiogenic leukemia.
The authors of many recent articles about radiation risk appear to be ignoring this evidence of a threshold. They do not challenge, endorse, comment on, or reference the recent publications that presented this evidence.
A colleague provided the following important comments on the 2015 article. The magnitude of the threshold is surprising high. Changing the value of the radiation dose in Zone C because of the severe pain of the leukemia patients creates misgivings. Supporting evidence is needed for the statement that radiation thresholds for other cancer types are expected to be higher than for leukemia. An explanation is needed for the omission of 42 years of follow-up leukemia data. The RERF data updates should be analyzed. Responses to these comments are given in the previous section.
The additional information in this article should remove the concerns that deter other scientists from accepting and referencing this evidence of a high threshold dose for radiation-induced leukemia. They may consider the possibility of higher thresholds for other cancer types.
A careful examination of Table 1 reveals the surprisingly low incidence of radiogenic leukemia among the atomic bomb survivors. It is only 0.5% of the population in the high radiation Zones A and B, shown in Table 1 (only 15 + 33 = 48 cases among 1241 + 8810 = 10 051 people).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. National Academy of Sciences (NAS)/National Research Council (NRC). The Biological Effects of Atomic Radiation (BEAR): a report to the public. NAS/NRC, Washington. 1956. Published as, Genetic effects of atomic radiation. Science. 1956;124(3209):1157–1164.17788880 [Google Scholar]
- 2. Cuttler JM. Remedy for radiation fear—discard the politicized science. Dose-Response. 2014;12(2):170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cuttler JM. Health effects of radiation exposures. Part B of intervenor report to Pickering NGS Public Hearing. Canadian Nuclear Safety Commission. CMD 18-H6-35 2018. http://www.nuclearsafety.gc.ca/eng/the-commission/hearings/cmd/pdf/cmd18-h6/CMD18-H6-35B.pdf. Accessed November 1, 2018.
- 4. Cameron JR. Longevity is the most appropriate measure of health effects of radiation. Radiology. 2003;229(1):14–15. https://pubs.rsna.org/doi/full/10.1148/radiol.2291030291. [DOI] [PubMed] [Google Scholar]
- 5. Sponsler R, Cameron JR. Nuclear shipyard worker study (1980-1988): a large cohort exposed to low-dose-rate gamma radiation. Int J Low Radiation. 2005;1(4):463–478. [Google Scholar]
- 6. Queens Cancer Center. Nasopharyngeal Radium Irradiation (NRI): fact Sheet; 2003. http://www.queenscancercenter.com/SpecificCancers/Brain/ReadingRoom/45,25786-1. Accessed November 1, 2018.
- 7. Calabrese EJ. LNTgate: how scientific misconduct by the U.S. NAS led to governments adopting LNT for cancer risk assessment. Environ Res. 2016;148:535–546. [DOI] [PubMed] [Google Scholar]
- 8. Calabrese EJ. LNTgate: the ideological history of cancer risk assessment. Tox Res Applic. 2017;1:1–3. [Google Scholar]
- 9. Calabrese EJ. The linear no-threshold (LNT) dose response model: a comprehensive assessment of its historical and scientific foundations. J Chemico Biological Interactions. 2018; In press. [DOI] [PubMed] [Google Scholar]
- 10. Jaworowski Z. Radiation hormesis—a remedy for fear. Human Exper Toxicol. 2010;29(4):263–270. http://journals.sagepub.com/doi/pdf/10.1177/0960327110363974. [DOI] [PubMed] [Google Scholar]
- 11. Wald N. Leukemia in Hiroshima city atomic bomb survivors. Science. 1958;127(3300):699–700. [DOI] [PubMed] [Google Scholar]
- 12. United Nations Scientific Committee on the Effects of Atomic Radiation. Report to the General Assembly New York, NY: United Nations; 1958. Annex G, p 165. Table VII. [Google Scholar]
- 13. Cuttler JM. Leukemia incidence of 96,000 Hiroshima atomic bomb survivors is compelling evidence that the LNT model is wrong. Arch Toxicol. 2014;88(3):847–848. [DOI] [PubMed] [Google Scholar]
- 14. Cuttler JM, Welsh JS. Leukemia and ionizing radiation revisited. J Leukemia. 2015;3:4 doi:10.4172/2329-6917.1000202. [Google Scholar]
- 15. Ulsh BA. A critical evaluation of the NCRP Commentary 27 endorsement of the linear no-threshold model of radiation effects. Environ Res. 2018;167:472–487. [DOI] [PubMed] [Google Scholar]
- 16. Doss M. Changing the paradigm of cancer screening, prevention and treatment. Dose Response. 2016;14(4):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakamoto K. Radiobiological basis for cancer therapy by total of half-body irradiation. Nonlinearity in Biology, Toxicology, and Medicine. 2004;2(4):293–316. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2657505/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metting N. 2010. Ionizing Radiation Dose Ranges (Sievert)http://www.dcfpnavymil.org/Library/tables/DoseRanges.pdf Office of Biological and Environmental Research. U.S. Department of Energy. Office of Science. [Google Scholar]
- 19. Fliedner TM, Graessle DH, Meineke V, Feinendegen LE. Hemopoietic response to low dose-rates of ionizing radiation shows stem cell tolerance and adaptation. Dose Response. 2012;10(4):644–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Richardson D, Sugiyama H, Nishi N, et al. Ionizing radiation and leukemia mortality among Japanese atomic bomb survivors, 1950-2000. Radiat Res. 2009;172(3):368–382. [DOI] [PubMed] [Google Scholar]
- 21. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. Chapter 10. [Google Scholar]


