Abstract
Background
Human immunodeficiency virus (HIV) is associated with diverse glomerular diseases. Characteristics of minimal change nephrotic syndrome (MCNS) in this setting have been little studied, and the specific features of this uncommon association remain to be determined.
Methods
We conduct a retrospective study. Clinical, biological and pathological characteristics of patients with MCNS and HIV infection were assessed. We evaluated HIV infection by in situ hybridization and CMIP expression by immunochemistry on kidney biopsies and compared it to HIV-associated nephropathy (HIVAN) and idiopathic MCNS.
Results
Eight patients were identifies. In all but one of these cases, MCNS occurred after HIV diagnosis (mean of 9.5 years). Acute kidney injury was detected in three cases. Mean CD4+ lymphocyte count was 733/mm3 and three patients had a detectable HIV viral load. In situ hybridization for HIV-1 RNA detection yielded a positive signal in a few tubular cells in the renal parenchyma in two of four patients with HIV infection associated with MCNS. Podocytes of these patients presented strong positive immunostaining for CMIP (4/4). Three patients suffered steroid-dependent nephrotic syndrome, and another two patients had at least one relapse. Rituximab treatment was initiated in four cases. After a median follow-up of 20 months, all patients were in remission (complete in 5 cases).
Conclusions
In patients with MCNS occurring in a context of HIV infection, podocyte injury seems to be associated with CMIP induction rather than renal HIV infection but further studies are needed to determine the molecular link between these two conditions.
Keywords: Minimal change nephrotic syndrome, HIV infection, AIDS, Podocytes, Albuminuria, C-mip, Rituximab
Background
Minimal change nephrotic syndrome (MCNS) is a glomerular disease characterized by massive selective proteinuria without glomerular lesions on light microscopy and with no immunoglobulin deposits on immunofluorescence study. MCNS has a higher incidence in children than in adults, but is, nevertheless, a frequent cause of nephrotic syndrome (NS) in adults, accounting for 10 to 25% of cases [1]. The pathogenesis of MCNS remains poorly understood, but there is compelling evidence, to suggest that it involves both immune system impairment and podocyte dysfunction [2]. In most cases, MCNS is considered to be an idiopathic glomerular disorder potentially triggered by immunological stimuli, such as viral infection, immunization or allergens [2]. MCNS may occur in association with chronic lymphoid neoplasms, such as Hodgkin disease [3], non-Hodgkin lymphoma [4] and thymoma [5], consistent with the hypothesis that it results from an immune system disorder. In the course of studies on molecular mechanisms underlying the pathophysiological processes involved in MCNS occurrence, we originally identified CMIP (c-maf inducing protein) as induced in T lymphocytes of patients with MCNS relapse but further studies have shown that CMIP is overproduced in podocytes of these patients, whereas it is downregulated in both tissues in MCNS remission [6, 7]. In addition, transgenic mice expressing selectively CMIP in podocytes develop heavy proteinuria without inflammatory lesions or immune complex deposits [6] and there is growing evidence that increase CMIP abundance could dramatically affect the function and survival of podocytes [2]. In rare cases, MCNS has also been reported in association with chronic viral infection [8, 9]. Human immunodeficiency virus (HIV) infection is a well-known cause of renal disease [10]. HIV-associated nephropathy (HIVAN) is the most common cause of glomerular injury in these patients [11]. A large number of studies have clearly demonstrated that HIVAN pathogenesis is triggered by the direct infection of glomerular epithelial cells and tubular epithelial cells by HIV [12]. In some studies, MCNS has been reported to account for 2–4% of all nephropathies associated with HIV [13, 14]. An extensive review of seven renal pathology studies including a total of 949 patients showed MCNS to be the main renal biopsy finding in 10 patients (1.05% of cases), suggesting that MCNS is a rare finding in HIV-infected patients [11]. It remains unclear whether there is a real pathophysiological link between MCNS and HIV infection, or just a fortuitous association.
In this study, we retrospectively identified patients with biopsy-proven MCNS and diagnosed with HIV infection, and we analyzed their clinical, histological, laboratory, and treatment data, to assess the significance of this rare association. We also performed in situ hybridization to determine whether HIV-RNA could be detected on renal biopsies from these patients and to assess possible CMIP expression, which has recently been shown to be associated with podocyte dysfunction [6].
Methods
Patients
We conducted a retrospective study, by sending a questionnaire to all French nephrology departments, to investigate the clinical, biological pathological and therapeutic data of adult patients with biopsy-proven MCNS in a context of HIV infection. At each hospital, patients were identified by searching renal pathology and clinical diagnosis databases. Eight patients were identified, for whom data were collected between 2000 and 2016, at the nephrology departments of eight French hospitals (Henri Mondor, Kremlin-Bicêtre, Pitié-Salpêtrière, Bichat, Tenon, Cannes, Clermont Ferrand and Tours). The study was performed in accordance with the ethical standards of the Helsinki Declaration, and has been approved by our local institutional review board.
Data were assessed for each patient at the time of MCNS diagnosis. The demographic and comorbid data collected included age, sex, ethnicity, hypertension, and coinfections with hepatitis B or hepatitis C viruses. Time from the diagnosis of HIV infection to the occurrence of MCNS, previous opportunistic infections, CD4+ T-cell count and HIV viral load were also recorded.
Highly active antiretroviral therapy (HAART) at the time of MCNS diagnosis was reported. All patients underwent renal biopsy for the exploration of a nephrotic syndrome, defined as a urinary protein excretion rate of more than 3 g/day and a serum albumin concentration below 30 g/L. MCNS diagnosis was confirmed by a pathological examination showing the presence of minimal change glomerular lesions on light microscopy, with no immunoglobulin and/or complement deposits in immunofluorescence study. Patients diagnosed with HIVAN on the basis of renal biopsy, or with focal and segmental glomerulosclerosis (FSGS) lesions without the typical pathological features of HIVAN were excluded. Acute kidney injury (AKI) was defined according to KDIGO (kidney disease improving global outcome) criteria [15]. Chronic kidney disease was defined as a permanent (lasting at least three months) decrease in estimated glomerular filtration rate (eGFR) to less than 60 mL/min/1.73 m2, according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [16]. Changes in HAART after MCNS diagnosis were systematically monitored. Complete remission (CR) of MCNS was defined as the return of urinary protein concentration to the normal range (< 0.3 g/24 h), together with an albumin level > 30 g/L. Relapse was defined as the recurrence of proteinuria in the nephrotic range for more than one week. Patients were considered to be in partial remission (PR) if proteinuria was between 0.3 and 3 g/day and albumin concentrations above 30 g/L. Steroid-dependent and steroid-resistant nephrotic syndromes were as previously defined by Hogan et al. [1].
In situ hybridization
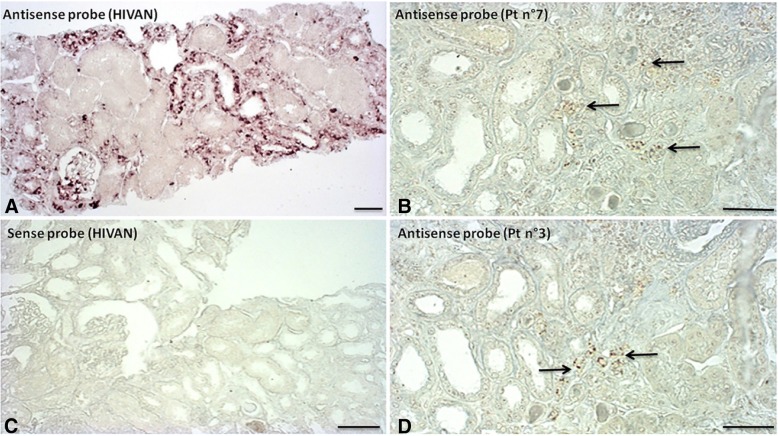
The potential localization of HIV infection in renal tissues affected by MCNS, as previously described [17], was investigated by in situ hybridization (ISH) for HIV-1 RNA on alcohol-formalin-acetic acid solution–fixed paraffin-embedded tissues from kidney biopsies. Labeling specificity was systematically checked by hybridizing sense probes with adjacent sections in parallel and antisense probes with uninfected renal tissues. For ISH, two patients with biopsy-proven HIVAN were used as positive controls (both have low CD4+ T-lymphocytes count < 200/mm3 and detectable viral load at the time of renal biopsy) and two patients with idiopathic MCNS as negative controls (initial episode in both cases).
Immunohistochemical analysis of CMIP expression
For immunohistochemistry-based analyses of CMIP expression, kidney samples were fixed for 16 h in Dubosq Brazil, then dehydrated and embedded in paraffin. Antigen retrieval was performed by immersing the slides in 0.01 mol/L citrate buffer and heating them in a 500 W microwave oven for 15 min. Endogenous peroxidase activity was blocked by incubation with 0.3% H2O2 in methanol for 30 min. Slides were incubated with blocking reagents containing avidin-biotin solution for 30 min and with normal blocking serum for 20 min, and were then incubated overnight with polyclonal anti-CMIP antibody at a final concentration of 2.5 μg/mL.
Results
Clinical and biological data for patients with MCNS occurring in a context of HIV infection
We retrospectively identified eight patients (three men and five women) with a diagnosis of MCNS and HIV infection. Their clinical and biological data at the time of renal biopsy are summarized in Table 1. Mean age at MCNS diagnosis was 43.3 years (range: 20 to 66 years) and five patients were of African ancestry. MCNS occurred after the diagnosis of HIV infection in seven cases, with an estimated mean interval of 9.5 years between these two events. In the remaining patient, the diagnosis of HIV infection coincided with MCNS (patient 8). All patients had typical features of MCNS at diagnosis, with nephrotic syndrome in all cases (mean proteinuria of 7.96 g/day (range: 3.33 to 14.8 g/day) and a mean albumin concentration of 17.5 g/L (7.7 to 29.5 g/L). None of them had MCNS resulting from a secondary process (lymphoid disorders or malignant disease) or potentially related to treatment known to be associated with MCNS occurrence (Lithium, Interferon, non-steroidal anti-inflammatory drugs). Three patients (37.5%) displayed AKI according to KDIGO criteria (stage 1 in two cases and stage 2 in one case). Four patients displayed microscopic hematuria during the first episode of MCNS (patients 2, 4, 5 and 7) and 1 patient (patients 3) presented microscopic hematuria during at least one relapse. At the time of renal biopsy, all but one (patient 8) of the patients had already received HAART, resulting in the successful control of CD4+ T-lymphocyte counts (mean CD4+ T-lymphocyte count of 733/mm3, range: 413/mm3 to 1028/mm3), but HIV was nevertheless detectable in three patients (patients 1, 7 and 8). As expected, HIV viral load was highest in patient 8, whose HIV infection was newly diagnosed (47,263 copies/mL). In this patient, HAART was not introduced immediately after HIV diagnosis because CD4+ T-lymphocytes count was normal (480/mm3).
Table 1.
Baseline characteristics of patients at the time of MCNS diagnosis
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Sex | F | M | F | F | F | M | F | M |
| Age (years) | 33 | 66 | 42 | 50 | 47 | 56 | 33 | 20 |
| Ethnicity | African | Caucasian | Caucasian | African | African | Caucasian | African | African |
| HT | No | No | No | No | No | Yes | No | No |
| Time between HIV diagnosis and MCNS (yr) | 7 | 7 | 22 | 9 | 4 | 23 | 4 | 0 |
| CD4+ T-cell count at MCNS diagnosis (/mm3) | 1028 | 738 | 1027 | 918 | 413 | 811 | 450 | 480 |
| HIV viral load at MCNS diagnosis (copies/ml) | 8029 | 0 | 0 | 0 | 0 | 0 | 1019 | 47,263 |
| Previous history of opportunistic infection | No | No | No | Pneumocystosis | No | No | M.avium pulmonary infection | No |
| HBV infection | No | Yes | No | No | No | Yes | Yes | No |
| HCV infection | No | No | No | No | No | Yes | No | No |
| Proteinuria (g/24 h) | 6.3 | 12 | 3.86 | 14.8 | 11 | 3.33 | 3.37 | 9 |
| Serum albumine (g/L) | 9.5 | 24 | 29.5 | 12 | 8 | 29 | 20 | 7.7 |
| Serum creatinine (μmol/L) | 109 | 130 | 77 | 62 | 110 | 61 | 134 | 79 |
| AKI stage (KDIGO) | 1 | 1 | No | No | No | No | 2 | No |
| Hematuria (cells/ml) | 0 | 34,000 | 0 | 34,000 | 13,000 | 0 | 500,000 | 0 |
F female, M male, HT hypertension, NA data not available, MCNS Minimal Change Nephrotic Syndrome, AKI acute kidney injury
The HAART regimen prescribed at the time of renal biopsy are listed in Table 2. MCNS diagnosis led to changes in HAART regimen in three patients (patients 2, 3 and 7). The main cause of modifications to the HAART regimen were the occurrence of AKI and prior administration of a HAART agent known to be potentially nephrotoxic (tenofovir in two patients).
Table 2.
Change in highly active antiretroviral therapy (HAART) following MCNS diagnosis
| Patient | HAART before MCNS diagnosis | Change in HAART at the time of MCNS occurrence |
|---|---|---|
| 1 | EVILTEGRAVIR COBICISTAT TENOFOVIR EMTRICITABINE |
No change |
| 2 | EMTRICITABINE TENOFOVIR ETRAVIRINE |
ABACAVIR LAMIVUDINE ETRAVIRINE |
| 3 | RALTEGRAVIR TENOFOVIR NEVIRAPINE |
RALTEGRAVIR NEVIRAPINE |
| 4 | EMTRICITABINE TENOFOIR RILPIVIRINE |
No change |
| 5 | LAMIVUDINE ZIDOVUDINE LOPINAVIR |
No change |
| 6 | RALTEGRAVIR ETRAVIRINE |
No change |
| 7 | ABACAVIR LOPINAVIR ETRAVIRINE |
ABACAVIR LAMIVUDINE DIDANOSINE LOPINAVIR |
| 8 | None | None (HAART introduction 4 years after diagnosis) |
In situ hybridization for HIV-1 RNA detection and immunohistochemistry studies of CMIP expression on renal biopsy specimens
Renal biopsy findings, including the results of light microscopy and immunofluorescence study were unremarkable. None of the eight patients had interstitial inflammatory infiltrate or acute tubular lesions. We investigated whether MCNS was associated with direct glomerular HIV infection, by analyzing HIV-1 RNA levels by in situ hybridization (ISH) on renal biopsies from four of the eight patients (patients 2, 3, 5, and 7). Unfortunately, renal biopsy specimens were not available for the 4 other patients. ISH with an antisense probe revealed the presence of HIV-1 RNA in the tubular and glomerular cells of control patients with HIVAN (n = 2), whereas no signal was detected with the sense probe (negative control) (Fig. 1 a and c). No HIV signal was obtained on renal biopsy specimens from control patients with idiopathic MCNS without HIV infection (n = 2) (data not shown). In two patients with MCNS occurring in a context of HIV infection (patients 2 and 5), no positive cells were detected on renal biopsies. By contrast, in the two others patients (patients 3 and 7), ISH with an antisense probe revealed the presence of HIV-1 RNA in a very small number of tubular cells (Fig. 1b and d), despite that viral load was below the detection threshold for patient 3. No signal was detected in podocytes.
Fig. 1.
Detection of HIV mRNA in renal biopsy specimens by in situ hybridization (ISH). Representative ISH of HIV-1 RNA with antisense and sense probe (negative control) for patients with HIVAN (a and c) and for two patients with MCNS occurring in a context of HIV infection (b and d). In patients with HIVAN, antisense probe hybridization yields a positive signal for tubular epithelial cells and some glomerular cells (1a). A sense riboprobe was used as a negative hybridization control in serial sections (1c). No staining was detected in patients with MCNS in the absence of HIV infection (data not shown), whereas rare positive tubular cells (arrows) were observed in the absence of glomerular staining in two of four patients with MCNS in a context of HIV infection (b and d). Scale bar, 50 μm
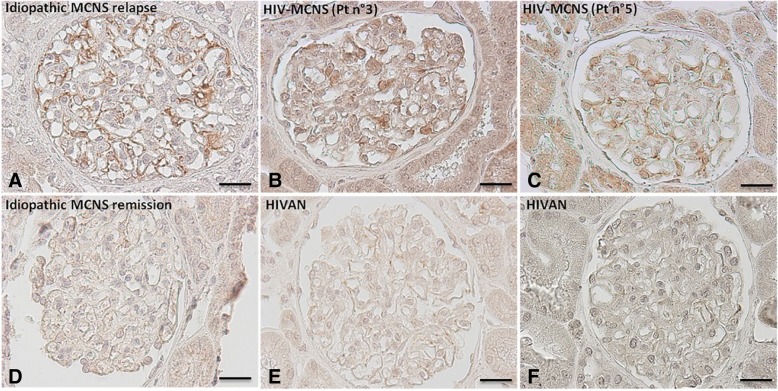
We then used immunohistochemistry methods to assess the expression of CMIP on renal biopsies from the same four patients with MCNS in a context of HIV infection. As observed in patient with idiopathic MCNS relapse on the renal biopsy performed during the initial episode (Fig. 2a), high levels of CMIP expression on podocytes were observed in patients with MCNS and HIV infection (Fig. 2b and c). By contrast, CMIP expression was very weak in the glomeruli of control patients with HIVAN, yielding a signal similar to that observed for a patient with MCNS in remission (Fig. 2 d, e and f).
Fig. 2.
CMIP expression on renal biopsy specimens from patients with MCNS in a context of HIV infection. CMIP is induced in the podocytes of patients with idiopathic MCNS relapse (biopsy at the time of the first episode) (a), but it is expressed at only very low levels during remission (d). Two representative cases from patients with MCNS in a context of HIV infection are shown, displaying strong staining with anti-CMIP antibody (b, c). By contrast, immunohistochemical studies of CMIP levels revealed very weak signals on the glomeruli of patients with HIVAN (e, f). Scale bar, 50 μm
Treatment and outcome of MCNS occurring in a context of HIV infection
At the time of MCNS diagnosis, six patients received steroids as a first-line treatment (Table 3). Five of these patients displayed CR of nephrotic syndrome, whereas no data were available for one patient, who was lost to follow-up (patient 7). Two patients displayed spontaneous remission without specific treatment (CR in patient 3 and PR in patient 6). Three of the patients successfully treated with steroid displayed steroid-dependent MCNS (patients 2, 4 and 5), necessitating long-term steroid therapy. Another two patients (patients 1 and 3) suffered at least one relapse during follow-up, with the first relapse occurring nine and eleven months, respectively, after MCNS diagnosis. One patient (patient 1) was successfully retreated with steroids alone for a single relapse. Second-line treatment was initiated in four patients, because of steroid dependence (patients 2, 4 and 5) or frequent relapses (patient 3). Rituximab was used for second-line treatment in three patients (patients 2, 3 and 4). Patient 3 displayed a spontaneous remission during the initial episode. During the first relapse, nephrotic syndrome occurred simultaneously with a cerebral thrombophlebitis requiring immediate therapeutic intervention. Nevertheless, she declined to be treated by high dose of steroids (1 mg/kg) due to potential side effects and clinicians decided to treat this relapse with low dose of steroids (0.5 mg/kg) in association with Rituximab administration. Subsequently this patient had two other relapse requiring Rituximab therapy. Patient 5 was also given rituximab, due to multiple relapses despite triple therapy including steroids, cyclosporine and mycophenolate mofetil (MMF). Rituximab treatment led to sustained remission in two patients (patients 2 and 4) whereas the other two patients (patients 3 and 5) suffered relapses seven and four months, respectively, after rituximab treatment. At the end of follow-up (median follow-up of 20 months; range: 9 to 111 months), all patients for whom data were available (7/8) were considered to be in remission (2 PR and 5 CR; Table 3), with a mean eGFR of 91 mL/min/1.73 m2. At the time of the last follow-up evaluation, four patients (patients 1, 2, 3 and 5) were on specific treatment for MCNS (steroids). Infectious episodes occurred in two patients on rituximab: pneumonia in patient 2, and two episodes of pyelonephritis and one of Clostridium difficile colitis in patient 3.
Table 3.
MCNS treatment and outcome
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| First-line treatment | Steroids | Steroids | Spontaneous remission | Steroids | Steroids | Spontaneous remission | Steroids | Steroids |
| CR | Y | Y | Y | Y | Y | N | NA | Y |
| PR | – | – | – | – | – | Y | NA | – |
| Steroid-dependence (dose of steroid at the time of relapse) | N | Y (10 mg/d) | N | Y (5 mg/d) | Y (5 mg/d) | N | NA | N |
| Steroid resistance | N | N | N | N | N | N | NA | N |
| Number of relapses during follow-up | 1 | 2 | 3 | 2 | > 6 | 0 | NA | 0 |
| Time between MCNS diagnosis and first relapse (months) | 9 | 3 | 11 | 1 | 8 | – | – | – |
| Second line treatment | Steroids | Steroids + Rituximab | Steroids + Rituximab | Steroids + Rituximab | Steroids + CsA + MMF + Rituximab | – | – | – |
| Follow-up (months) | 13 | 20 | 68 | 14 | 111 | 9 | NC | 91 |
| Status at last follow-up visit | CR | PR | CR | CR | CR | PR | NA | CR |
| Proteinuria (g/24 h) at last follow-up visit | 0.1 | 1.25 | 0.12 | 0.1 | 0.28 | 0.89 | NA | 0.1 |
| Serum albumine (g/L) at last follow-up visit | 36.2 | 37.2 | 40 | 37 | 31 | 43 | NA | 40 |
| Serum creatinine (μmol/l) at last-follow-up visit | 78 | 95 | 138 | 64 | 66 | 71 | NA | 109 |
| eGFR CKD-EPI (ml/min/1.73m2) at last follow-up visit | 99 | 73 | 39 | 104 | 120 | 99 | NA | 108 |
Y Yes, N No, CsA cyclosporine A, CR complete remission, PR partial remission, NA data not available
Discussion
HIV-related renal diseases include a large diversity of pathological entities, but the most relevant glomerular disorders observed in these patients are HIVAN and immune complex-mediated glomerular disease [11, 18]. The occurrence of MCNS in the setting of HIV infection has never been studied in detail and the main characteristics of this association remain to be determined. Over a 16-year period, we could find only eight patients with MCNS in a context of HIV infection, suggesting that this association may be fortuitous rather than indicative of a direct relationship. However, our study was not designed to accurately assess the prevalence of MCNS in HIV infected patients.
Genetic factors, including APOL1 variants, have recently emerged as key factors potentially increasing susceptibility to HIVAN in HIV-infected patients of African ancestry [19, 20]. In our study, APOL1 variants were not systematically checked, but the distribution of Caucasian and African patients in our cohort suggests that this was not a susceptibility factor for this association. Moreover a recent study suggested that two risk allele genotype is uncommon in patients with MCNS and mainly found in patient with FSGS steroid resistant nephrotic syndrome [21]. In a retrospective study, Lescure et al. described changes in the pattern of glomerular lesions in HIV patients [14]. They demonstrated that the incidence of HIVAN had strongly decreased over time, since the introduction of HAART, and that classical FSGS was the leading causes of glomerular disease in HIV-infected patients. Lescure et al. found that MCNS was the main pathological lesion in four patients (4.5% of cases). Strikingly, seven patients from our cohort were on HAART and HIV infection was considered to be under control at the onset of nephrotic syndrome. These data indicate that MCNS was probably not due to severe immunodeficiency in these patients. MCNS occurred exclusively in one patient newly diagnosed with HIV infection, with no obvious difference in clinical, biological and histological presentation compared to other patients in whom HIV infection was well controlled. In this specific case, we can not rule out that HIV infection in the context of immune susceptibility or particular genetic background might contribute to trigger MCNS relapse, as was recently reported for EBV infection [22]. In three patients, the diagnosis of MCNS led to change the antiretroviral drugs used, due to potential tenofovir-mediated renal toxicity in two cases and AKI in one case. Tenofovir administration has been identified as a potential cause of tubular injury, sometimes associated with significant but non-nephrotic proteinuria [23]. However, none of our patients on this drug had typical proximal tubular dysfunction or pathological lesions suggestive of toxic tubular necrosis. In a study of 95 adult patients with idiopathic MCNS, Waldman et al. found that AKI was present at initial presentation or during a relapse episode in 25.2% of cases, mostly due to acute tubular injury or interstitial inflammation [24]. Three patients from our cohort displayed AKI according to KDIGO criteria but, in these cases, analyses of renal biopsies provided no evidence of interstitial inflammatory lesions or tubular injury, suggesting a prerenal origin of AKI.
We investigated the potential role of HIV infection in MCNS occurrence in this context, by performing ISH to determine whether the renal cells of these patients were infected. By contrast to HIVAN patients, in which large numbers of tubular and glomerular cells gave a positive signal, no HIV RNA was detected in the cells of two patients from our cohort with viral loads below the threshold of detection. Nevertheless, ISH detected a few tubular cells displaying positive staining, with a complete absence of staining in the glomerular area, in two other patients with MCNS in a context of HIV infection. In one case, HIV RNA was detected in the renal parenchyma, despite undetectable viremia. Winston et al. were the first to demonstrate, in a patient diagnosed with HIVAN that HIV-1 mRNA may persist in the renal epithelium even if viral RNA is undetectable in the plasma, following the initiation of HAART [25]. Consistent with this hypothesis, Canaud et al. found that kidney graft parenchyma may serve as a viral reservoir after kidney transplantation [17]. Another key finding of our study is the induction of CMIP expression in the podocytes of patients with MCNS in a context of HIV infection. CMIP upregulation has been described in renal biopsies from patients with idiopathic MCNS, FSGS and membranous nephropathy, whereas CMIP is not generally expressed in inflammatory and proliferative glomerular diseases, such as IgA nephropathy or active lupus nephritis [6, 26, 27]. In vivo and in vitro experiments have shown that CMIP plays a crucial role in podocyte dysfunction, by inactivating the nephrin and Akt signaling pathways responsible for altering podocyte signaling and cytoskeleton remodeling, an important event in the induction of proteinuria [6]. In addition, experimental data from several models of podocyte injury suggest that targeting CMIP with specific inhibitors taken up by podocytes may be a promising future therapeutic approach for some glomerular diseases associated with the overproduction of CMIP in podocytes [6, 26]. By contrast, in patients with HIV infection, pathophysiological processes involved in HIVAN seems to be closely related to viral integration into target cells. Thus, the expression of HIV proteins, such as Nef, in particular, in podocytes, leads to the activation of STAT3 signaling, which seems to be a key factor in HIVAN pathogenesis [28, 29]. Thus, the absence of HIV RNA in glomerular cells and the induction of CMIP in podocytes from patients with MCNS in a context of HIV infection suggest that the occurrence of MCNS in individuals with HIV infection may be coincidental and unrelated. As MCNS may primarily result from an immune system disorder [2], chronic HIV infection may triggers immune disturbances in some patients, promoting podocyte dysfunction.
In accordance with current recommendations for the treatment of initial episodes of idiopathic MCNS in adult patients [1], first-line treatment with steroids was initiated in six patients, leading to CR in five cases (the remaining patient being lost to follow-up). Two of the patients in our cohort presented spontaneous remission, consistent with the findings of Maas et al., who reported the occurrence of spontaneous progressive remission in 10% of patients not receiving specific therapy [30]. After a median of 20 months of follow-up, five of the seven patients for whom data were available presented relapses of MCNS (including three patients with steroid-dependent MCNS). Strikingly, rituximab was the treatment of choice most frequently prescribed for patients with steroid-dependent MCNS. It was also prescribed for one patient with frequent relapses. Rituximab has recently emerged as a promising treatment for steroid-dependent nephrotic syndrome [31–33]. In a context of HIV infection, Rituximab is primary used for the treatment of patients with HIV related lymphoma and Multicentric Castleman’s disease [34]. However, Rituximab may increase the risk of infectious episodes in HIV patients. Our retrospective study was not designed to investigate the safety and tolerance of Rituximab in this population. In our cohort, non-fatal bacterial infections occurred in two patients on rituximab. Given that rituximab administration has been associated with an increase in the risk of hepatitis B reactivation, opportunistic infections and polyomavirus JC-related progressive multifocal leukoencephalopathy [35], this treatment should probably be used with caution in HIV-infected patients.
Conclusions
MCNS may be observed in HIV patients, but seems to be a rare glomerular disease in these patients. Our pathology studies suggested that HIV infection was not directly involved in podocyte dysfunction and that CMIP induction, through unknown mechanisms, might play a key role in podocyte dysfunction and the occurrence of proteinuria. First-line treatment for MCNS in patients with HIV infection should include steroids, but, given the high incidence of MCNS relapse and steroid dependence, the potential contribution of rituximab to treatment should be investigated further.
Acknowledgments
We would like to acknowledge all the renal pathologists who assessed the kidney biopsies of the patients included in the present study.
Funding
No funding was received for this study.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- AKI
Acute kidney injury
- APOL1
Apolipoprotein L1
- CKD-EPI
Chronic kidney disease epidemiology collaboration
- CMIP
C-maf induction protein
- CR
Complete remission
- eGFR
estimated glomerular filtration rate
- FSGS
Focal segmental glomerulosclerosis
- HAART
Highly active anti retroviral therapy
- HIV
Human immunodeficiency virus
- HIVAN
Human immunodeficiency virus associated nephropathy
- ISH
In situ hybridization
- KDIGO
Kidney disease improving global outcome
- MCNS
Minimal change nephrotic syndrome
- MMF
Mycophenolate mofetil
- NS
Nephrotic syndrome
- PR
Partial remission
- RNA
Ribonucleic acid
Authors’ contribution
All authors have read and approved the manuscript. RA, DS and VA designed the research study, collected and analyzed the data and wrote the manuscript, ND-R, AP-S and GC performed in situ hybridization study and SY-Z performed immunohistochemistry study. EP, C I-B, PG, QR, DB, FV, A-E H, HF, AM, GC, MM, IB collected the data, provided clinical and pathological information and critically reviewed the manuscript. VA takes responsibility that this study has been reported honestly, accurately, and transparently.
Ethics approval and consent to participate
The study was performed in accordance with the ethical standards of the Helsinki Declaration, and has been approved by our local institutional review board: IRB Mondor n°00003835. All patients included in this study have given their written consent to perform if required a complementary investigation on their renal biopsy specimen and for the use of their personal data (data anonymization) after a declaration to the CNIL (French National Comission for Liberties and Informatic).
Consent for publication
Patients have given their written consent to have their clinical and biological data published in an anonymized form.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Romain ARRESTIER, Phone: +33149814446, Email: arrestier_romain@yahoo.fr.
Anne-Pascale SATIE, Email: anne-pascale.satie@univ-rennes1.fr.
Shao-yu ZHANG, Email: shao-yu.zhang@inserm.fr.
Emmanuelle PLAISIER, Email: emmanuelle.plaisier@aphp.fr.
Corinne ISNARD-BAGNIS, Email: corinne.bagnis@psl.aphp.fr.
Philippe GATAULT, Email: philippe.gatault@univ-tours.fr.
Quentin RAIMBOURG, Email: quentin.raimbourg@aphp.fr.
David BUOB, Email: david.buob@aphp.fr.
Flavia VOCILA, Email: f.vocila@ch-cannes.fr.
Anne-Elisabeth HENG, Email: aheng@chuclermontferrand.fr.
Helene FRANCOIS, Email: helene.francois@aphp.fr.
Anissa MOKTEFI, Email: anissa.moktefi@aphp.fr.
Guillaume CANAUD, Email: guillaume.canaud@inserm.fr.
Marie MATIGNON, Email: marie.matignon@aphp.fr.
Nathalie DEJUCQ-RAINSFORD, Email: nathalie.dejucq-rainsford@inserm.fr.
Isabelle BROCHERIOU, Email: isabelle.brocheriou@aphp.fr.
Dil SAHALI, Email: dil.sahali@inserm.fr.
Vincent AUDARD, Email: vincent.audard@aphp.fr.
References
- 1.Hogan J, Radhakrishnan J. The treatment of minimal change disease in adults. J Am Soc Nephrol JASN. 2013;24:702–711. doi: 10.1681/ASN.2012070734. [DOI] [PubMed] [Google Scholar]
- 2.Sahali D, Sendeyo K, Mangier M, Audard V, Zhang SY, Lang P, et al. Immunopathogenesis of idiopathic nephrotic syndrome with relapse. Semin Immunopathol. 2014;36:421–429. doi: 10.1007/s00281-013-0415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Audard V, Zhang S, Copie-Bergman C, Rucker-Martin C, Ory V, Candelier M, et al. Occurrence of minimal change nephrotic syndrome in classical Hodgkin lymphoma is closely related to the induction of c-mip in Hodgkin-reed Sternberg cells and podocytes. Blood. 2010;115:3756–3762. doi: 10.1182/blood-2009-11-251132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kofman T, Zhang S-Y, Copie-Bergman C, Moktefi A, Raimbourg Q, Francois H, et al. Minimal change nephrotic syndrome associated with non-Hodgkin lymphoid disorders: a retrospective study of 18 cases. Medicine (Baltimore) 2014;93:350–358. doi: 10.1097/MD.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karras Alexandre, de Montpreville Vincent, Fakhouri Fadi, Grünfeld Jean-Pierre, Lesavre Philippe. Renal and thymic pathology in thymoma-associated nephropathy: report of 21 cases and review of the literature. Nephrology Dialysis Transplantation. 2005;20(6):1075–1082. doi: 10.1093/ndt/gfh615. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S-Y, Kamal M, Dahan K, Pawlak A, Ory V, Desvaux D, et al. C-mip impairs podocyte proximal signaling and induces heavy proteinuria. Sci Signal. 2010;3:ra39. doi: 10.1126/scisignal.2000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimbert P, Valanciute A, Audard V, Pawlak A, Le gouvelo S, Lang P, et al. Truncation of C-mip (Tc-mip), a new proximal signaling protein, induces c-maf Th2 transcription factor and cytoskeleton reorganization. J Exp Med. 2003;198:797–807. doi: 10.1084/jem.20030566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aoyama M, Sugimoto T, Yokono T, Sakaguchi M, Deji N, Uzu T, et al. Minimal-change nephropathy and chronic hepatitis C infection: coincidental or associated? Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2007;22:1479–1480. doi: 10.1093/ndt/gfl808. [DOI] [PubMed] [Google Scholar]
- 9.Zhou T-B, Jiang Z-P. Is there an association of hepatitis B virus infection with minimal change disease of nephrotic syndrome? A clinical observational report. Ren Fail. 2015;37:459–461. doi: 10.3109/0886022X.2014.1001711. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SD, Kopp JB, Kimmel PL. Kidney diseases associated with human immunodeficiency virus infection. N Engl J Med. 2017;377:2363–2374. doi: 10.1056/NEJMra1508467. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg AZ, Naicker S, Winkler CA, Kopp JB. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol. 2015;11:150–160. doi: 10.1038/nrneph.2015.9. [DOI] [PubMed] [Google Scholar]
- 12.Ross MJ. Advances in the pathogenesis of HIV-associated kidney diseases. Kidney Int. 2014;86:266–274. doi: 10.1038/ki.2014.167. [DOI] [PubMed] [Google Scholar]
- 13.Lopes GS, Marques LP, Rioja LS, Basilio-de-Oliveira CA, Oliveira AV, Nery AC, et al. Glomerular disease and human immunodeficiency virus infection in Brazil. Am J Nephrol. 1992;12:281–287. doi: 10.1159/000168461. [DOI] [PubMed] [Google Scholar]
- 14.Lescure F.-X., Flateau C., Pacanowski J., Brocheriou I., Rondeau E., Girard P.-M., Ronco P., Pialoux G., Plaisier E. HIV-associated kidney glomerular diseases: changes with time and HAART. Nephrology Dialysis Transplantation. 2012;27(6):2349–2355. doi: 10.1093/ndt/gfr676. [DOI] [PubMed] [Google Scholar]
- 15.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Supp. 2012;l2:1–138.
- 16.Kidney Disease Improving global outcomes (KDIGO) CKD work group: KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 17.Canaud G, Dejucq-Rainsford N, Avettand-Fenoël V, Viard J-P, Anglicheau D, Bienaimé F, et al. The kidney as a reservoir for HIV-1 after renal transplantation. J Am Soc Nephrol JASN. 2014;25:407–419. doi: 10.1681/ASN.2013050564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nobakht E, Cohen SD, Rosenberg AZ, Kimmel PL. HIV-associated immune complex kidney disease. Nat Rev Nephrol. 2016;12:291–300. doi: 10.1038/nrneph.2015.216. [DOI] [PubMed] [Google Scholar]
- 19.Fine DM, Wasser WG, Estrella MM, Atta MG, Kuperman M, Shemer R, et al. APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol JASN. 2012;23:343–350. doi: 10.1681/ASN.2011060562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasembeli AN, Duarte R, Ramsay M, Mosiane P, Dickens C, Dix-Peek T, et al. APOL1 risk variants are strongly associated with HIV-associated nephropathy in black south Africans. J Am Soc Nephrol JASN. 2015;26:2882–2890. doi: 10.1681/ASN.2014050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gribouval O, Boyer O, Knebelmann B, Karras A, Dantal J, Fourrage C, et al. APOL1 risk genotype in European steroid-resistant nephrotic syndrome and/or focal segmental glomerulosclerosis patients of different African ancestries. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2018. 10.1093/ndt/gfy176. [DOI] [PubMed]
- 22.Dossier C, Sellier-Leclerc A-L, Rousseau A, Michel Y, Gautheret-Dejean A, Englender M, et al. Prevalence of herpesviruses at onset of idiopathic nephrotic syndrome. Pediatr Nephrol Berl Ger. 2014;29:2325–2331. doi: 10.1007/s00467-014-2860-1. [DOI] [PubMed] [Google Scholar]
- 23.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D’Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78:1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 24.Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol CJASN. 2007;2:445–453. doi: 10.2215/CJN.03531006. [DOI] [PubMed] [Google Scholar]
- 25.Winston JA, Bruggeman LA, Ross MD, Jacobson J, Ross L, D’Agati VD, et al. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med. 2001;344:1979–1984. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- 26.Sendeyo K, Audard V, Zhang S, Fan Q, Bouachi K, Ollero M, et al. Upregulation of c-mip is closely related to podocyte dysfunction in membranous nephropathy. Kidney Int. 2013;83:414–425. doi: 10.1038/ki.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ory V, Fan Q, Hamdaoui N, Zhang S, Desvaux D, Audard V, et al. C-mip down-regulates NF-κB activity and promotes apoptosis in podocytes. Am J Pathol. 2012;180:2284–2292. doi: 10.1016/j.ajpath.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 28.He JC, Husain M, Sunamoto M, D’Agati VD, Klotman ME, Iyengar R, et al. Nef stimulates proliferation of glomerular podocytes through activation of Src-dependent Stat3 and MAPK1,2 pathways. J Clin Invest. 2004;114:643–651. doi: 10.1172/JCI200421004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng X, Lu T-C, Chuang PY, Fang W, Ratnam K, Xiong H, et al. Reduction of Stat3 activity attenuates HIV-induced kidney injury. J Am Soc Nephrol JASN. 2009;20:2138–2146. doi: 10.1681/ASN.2008080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maas RJ, Deegens JK, Beukhof JR, Reichert LJ, Ten Dam MA, Beutler JJ, et al. The clinical course of minimal change nephrotic syndrome with onset in adulthood or late adolescence: a case series. Am J Kidney Dis Off J Natl Kidney Found. 2017;69:637–646. doi: 10.1053/j.ajkd.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Lond Engl. 2014;384:1273–1281. doi: 10.1016/S0140-6736(14)60541-9. [DOI] [PubMed] [Google Scholar]
- 32.Munyentwali H, Bouachi K, Audard V, Remy P, Lang P, Mojaat R, et al. Rituximab is an efficient and safe treatment in adults with steroid-dependent minimal change disease. Kidney Int. 2013;83:511–516. doi: 10.1038/ki.2012.444. [DOI] [PubMed] [Google Scholar]
- 33.Boumediene Ahmed, Vachin Pauline, Sendeyo Kelhia, Oniszczuk Julie, Zhang Shao-yu, Henique Carole, Pawlak Andre, Audard Vincent, Ollero Mario, Guigonis Vincent, Sahali Djillali. NEPHRUTIX: A randomized, double-blind, placebo vs Rituximab-controlled trial assessing T-cell subset changes in Minimal Change Nephrotic Syndrome. Journal of Autoimmunity. 2018;88:91–102. doi: 10.1016/j.jaut.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Reid E, Nooka A, Blackmon J, Lechowicz MJ. Clinical use of rituximab in patients with HIV related lymphoma and multicentric Castleman’s disease. Curr Drug Deliv. 2012;9:41–51. doi: 10.2174/156720112798376005. [DOI] [PubMed] [Google Scholar]
- 35.Kronbichler A, Windpessl M, Pieringer H, Jayne DRW. Rituximab for immunologic renal disease: what the nephrologist needs to know. Autoimmun Rev. 2017;16:633–643. doi: 10.1016/j.autrev.2017.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.