Abstract
Purpose:
Aneurysmal bone cyst (ABC) is a rare skeletal tumor usually treated with surgery/embolization. We hypothesized that owing to similarities with giant cell tumor of bone (GCTB), denosumab was active also in ABC.
Methods:
In this observational study, a retrospective analysis of ABC patients treated with denosumab was performed. Patients underwent radiologic disease assessment every 3 months. Symptoms and adverse events were noted.
Results:
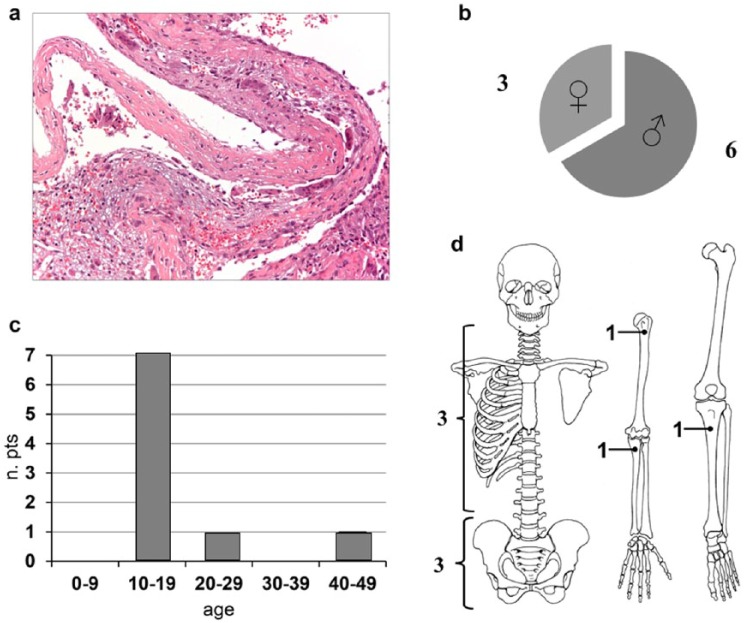
Nine patients were identified (6 male, 3 female), with a median age of 17 years (range 14–42 years). Primary sites were 6 spine–pelvis, 1 ulna, 1 tibia, and 1 humerus. Patients were followed for a median time of 23 months (range 3–55 months). Patients received a median of 8 denosumab administrations (range 3–61). All symptomatic patients had pain relief and 1 had paresthesia improvement. Signs of denosumab activity were observed after 3 to 6 months of administration: bone formation by computed tomography scan was demonstrated in all patients and magnetic resonance imaging gadolinium contrast media decrease was observed in 7/9 patients. Adverse events were negligible. At last follow-up, all patients were progression-free: 5 still on denosumab treatment, 2 off denosumab were disease-free 11 and 17 months after surgery, and the last 2 patients reported no progression 12 and 24 months after denosumab interruption and no surgery.
Conclusions:
Denosumab has substantial activity in ABCs, with favorable toxicity profile. We strongly support the use of surgery and/or embolization for the treatment of ABC, but denosumab could have a role as a therapeutic option in patients with uncontrollable, locally destructive, or recurrent disease.
Keywords: Aneurysmal bone cyst, denosumab, bone tumor, receptor activator of nuclear factor-kappa B ligand
Introduction
Aneurysmal bone cysts (ABCs) are benign bone tumors with a peak age at incidence in the first 2 decades of life.1-4 ABCs usually present with a growing mass, swelling, pain, and bone destruction1-4; in some cases, the lesion is locally aggressive and might be associated with pathologic fractures.2,5 ABCs can involve metaphysis of appendicular bones and axial bones. In case of spinal localization, neurologic deficit may be caused by infiltration and compression of nerve roots.4,6-10 ABCs can occur as a primary tumor in about 70% of cases or as a secondary tumor in 30% of cases; the latter can be associated with osteoblastoma, giant cell tumor of bone (GCTB), chondroblastoma, fibrous dysplasia, or low-grade osteosarcoma.1-4 Historically, the etiology of the lesion was attributed to an increase of venous vascular pressure in the bone, resulting in dilation of small vessels that lead to reabsorption of the matrix.2 Recent studies have shown chromosomal rearrangements, such as translocations, resulting in the upregulation of USP6 gene.2,5,11-15 USP6 arrests the normal maturation of osteoblasts and increases the production of matrix metalloproteinase.12 ABCs present as an expansive osteolytic lesion on X-rays, while magnetic resonance imaging (MRI) often shows septate cystic cavities with fluid-fluid levels due to blood sedimentation.1-5 Histopathologically, the lesions consist of a blood-filled cavity separated by fibrous septa not lined by endothelial cells and composed of spindle cells, inflammatory cells, and multinucleated giant cells (MNGCs) 2 (Figure 1). Nuclear atypia is not present.2 Biopsy is mandatory to exclude telangiectatic osteosarcoma2,16 and differential diagnoses with other lesions characterized by the presence of osteoclastic giant cells: giant cell tumor, brown tumor, and nonossifying fibroma.5 Treatment options for ABCs are represented by surgical resection or curettage, with bone graft or cement usually used to fill the defect,1,2,6,7,17,18 selective arterial embolization,2,6,8,9,19 sclerotherapy,2,20-22 or radiotherapy.23 However, all these therapeutic options are burdened with complications2,4,10,15,23 and innovative therapies are needed to treat ABCs.24
Figure 1.
Blood-filled cavity separated by fibrous septa composed of spindle cells, inflammatory cells, multinucleated osteoclast-like giant cells, and fibroblast-like cells. Nuclear atypia is not present (a); gender distribution (b); age (c); tumor site (d).
Denosumab is a human monoclonal antibody that binds the cytokine receptor activator of nuclear factor-kappa B ligand (RANKL), which essentially initiates bone turnover.11 RANKL inhibition blocks osteoclast maturation and function,11 and denosumab has been successfully used in the treatment of osteoporosis,25 skeletal metastases,26 and more recently GCTB as well.27 The satisfactory results with denosumab in the treatment of GCTB,28 the immunohistochemical similarity and relationship between GCTB and ABCs,29 and the activity of denosumab in a few cases of ABC29 justify the hypothesis that denosumab may also have positive effects for ABC patients. Few series are reported in the literature about the results of the treatment of ABCs with denosumab.14,29,30
The aim of this study was to evaluate the clinical and radiologic response of patients with ABCs treated with denosumab in case of inoperable tumors or when surgery was feasible, but associated with severe morbidity.
Methods
We performed a retrospective analysis of ABC patients treated off-label with denosumab, due to the impossibility to perform surgical treatment, when surgery was associated with severe morbidity, or when arterial embolization failed due to the absence of appropriate afferent arteries. The study was approved by the appropriate institutional review committee and meets the national guidelines. Denosumab was administered as a subcutaneous injection in the dose of 120 mg on days 1, 8, 15, 29, and every 4 weeks thereafter. To prevent hypocalcaemia, a daily supplementation of calcium 500 mg and vitamin D 400 IU was administered.9 Computed tomography (CT) scan and/or MRI disease assessment was performed at 3, 6, 9, and 12 months for all patients. All images were centralized and reviewed for the purpose of this study, with radiologic review not blinded to clinical information.
Denosumab was discontinued on the subject’s decision to withdraw, for adverse side effects, or if the lesion was surgically removed. All clinical data were recorded from patient charts. We collected information on pain, symptoms, and adverse events associated with denosumab. Histologic response was assessed in patients undergoing surgical removal.
Results
From October 2012 to July 2015, 9 patients (6 male and 3 female) treated with denosumab for ABCs were identified. The median age was 17 years (range 14–42 years). The primary site was spine/pelvis in 6 cases, and ulna, tibia, and humerus in 1 case each. Two patients were surgically treated after preoperative denosumab administration. With a median follow-up of 23 months (range 3–55 months), patients received a median and mean of 8 and 21 denosumab administrations, respectively (range 3–61).
One patient was asymptomatic, whereas clinical improvement was observed in the remaining 8 patients: 7 patients with pain had relief/decrease of symptoms on the 11-point scale and 1 patient had paresthesia improvement. The radiologic outcome showed good responses after 3 to 6 months of denosumab: CT scan showed bone formation in all patients and MRI gadolinium contrast media decrease was seen in 7/9 patients. Sustained tumor control was demonstrated in all patients (RECIST does not apply, due to disease site within bone and substantial denosumab-induced calcification) (Figures 2–4).
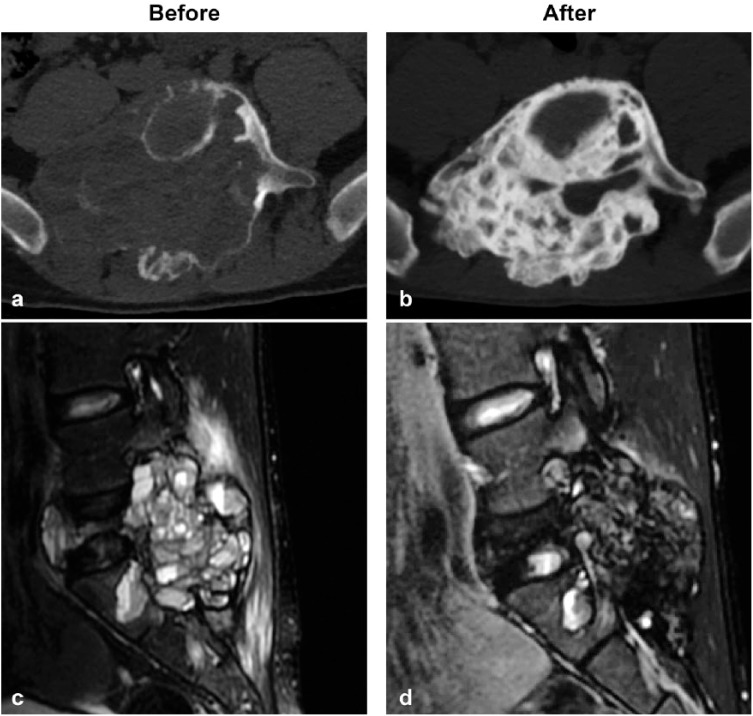
Figure 2.
Denosumab-induced bone calcification on computed tomography scan (a, b) and contrast medium decrease on magnetic resonance imaging (c, d) in a 16-year-old boy with spine aneurysmal bone cyst. Baseline (a, c) and after 33 administrations of denosumab 120 mg (b, d).
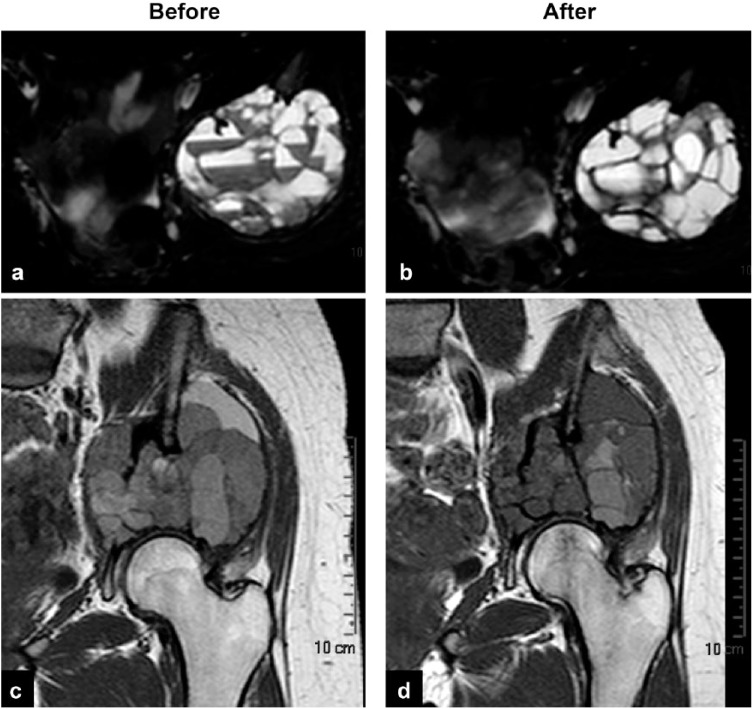
Figure 3.
A 16-year-old girl with pelvic aneurysmal bone cyst presenting fluid–blood levels and contrast medium enhancement at baseline (a, c), both reduced after 9 administrations of denosumab (b, d).
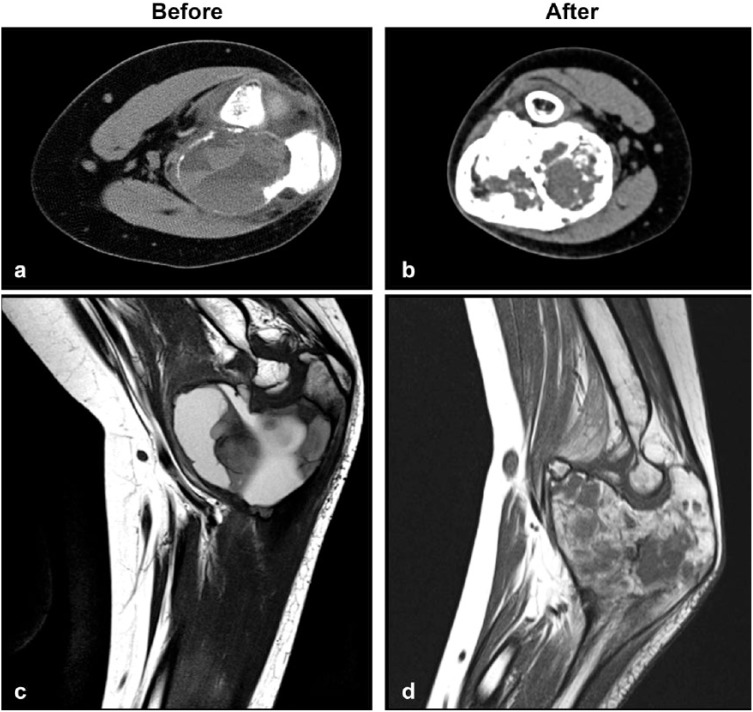
Figure 4.
A 12-year-old boy presenting with ulnar aneurysmal bone cyst: fluid–blood levels and cortical disruption at baseline (a, c), responding after 6 administrations of denosumab (b, d).
Two patients, a 19-year-old man and a 17-year-old girl with proximal humerus and distal tibia ABCs, underwent surgery after 5 and 9 months of denosumab treatment. The humeral lesion had relapsed after previous surgery and sclerosants (3% polidocanol injection). Both lesions underwent curettage and bone filling. After curettage, the cavity was first filled with a gel and frozen with argon-helium cryoprobes (Endocare) as local adjuvant treatment; then bone grafting with morcelized allografts was performed. A plate fixation was applied at the humeral lesion; no fixation was performed at the tibia. Histologic complete response (i.e., disappearance of giant cell component) was demonstrated in both cases (Figure 5).
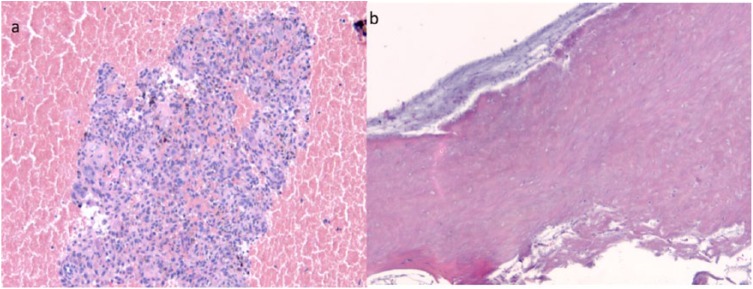
Figure 5.
Biopsy of a distal tibia aneurysmal bone cyst in a 17-year-old girl pre-denosumab. (a) Abundant blood is present at periphery and giant cells in the context of high cellularity lesion rich in mononuclear cells in the central area. (b) Post-denosumab: disappearance of all mononucleated and multinucleated cells with trabecular and hyaline cells (denosumab-induced changes).
Side effects were observed in only one patient, who presented grade 1 vomiting (Table 1). None of the patients developed osteonecrosis of jaw (ONJ), nor were abnormal laboratory results observed.
Table 1.
Denosumab in the treatment of patients with aneurysmal bone cyst.
| Authors | Year | No. of patients | Age, y/sex | Site | Symptoms | Clinical improvement | Bone formation | Complications |
|---|---|---|---|---|---|---|---|---|
| Pelle et al.31 | 2013 | 1 | 5/M | Sacrum | Pain, bowel incontinence, urinary retention | Yes | Yes | No |
| Lange et al.24 | 2013 | 2 | 8/M | C5 | Pain, radiculopathy, paresis | Yes (pain decrease) | Yes | Asymptomatic hypocalcemia |
| 11/M | C5 | — | — | Yes | No | |||
| Pauli et al.5 | 2014 | 1 | 21/F | Forearm | Swelling, pain | Yes | Yes | No |
| Our series | 2017 | 9 | 14/F | Sacrum | — | — | Yes | No |
| 16/M | L5-S1 | Pain | Yes | Yes | No | |||
| 42/M | Spine | Paresthesia | Yes | Yes | No | |||
| 16/F | Iliac wing | Pain | Yes | Yes | No | |||
| 12/M | Proximal ulna | Pain | Yes | Yes | No | |||
| 19/M | Proximal humerus | Pain | Yes | Yes | No | |||
| 17/F | Distal tibia | Pain | Yes | Yes | No | |||
| 25/M | Spine | Pain, radiculopathy | Yes | Yes | Vomiting, grade 1 | |||
| 19/M | Spine | Pain | Yes | Yes | No |
At last follow-up, all patients were progression-free: 5 patients were still on denosumab treatment, 2 patients were disease-free 11 and 17 months after the curettage, while in the last 2 cases, 12- and 16-year-old patients, discontinuing denosumab after 8 months and 3 years, respectively, no progression was documented 12 and 24 months after denosumab interruption and no surgery.
Discussion
ABCs are benign tumors with potential locally aggressive behavior, characterized by loculated blood-filled cystic areas. ABC lesions contain osteoclast-like MNGCs and fibroblast-like cells,1,24,31,32 similarly to GCTB.24 Since osteoclasts are the only cells responsible for bone resorption, the MNGCs within GCTB and ABCs appear to be responsible for the osteolytic natures of these tumors.33
Also, similar to GCTB, RANKL is highly expressed in stroma of ABCs and dictates the activation of MNGCs,34 binding to RANK present on the surface of monocyte and macrophage lineage precursors.14,24-28,30,35,36 The RANK signalling pathway has an essential role in tumor progression.31
Several local treatment options might be used for ABCs, including surgery, embolization, sclerotherapy, and radiotherapy.1-3,6-9,17-19,22,29 Open surgery is considered the gold standard for the treatment of ABCs, with a local control rate up to 100%.7,17,18 However, the complication rate is not negligible,2,4 particularly in case of spinal ABCs, which are associated with a high risk of morbidity such as neurologic impairment, instability, recurrence, or other vital problems.4,6-10 Moreover, the intense vascularization of ABC lesions can result in significant intraoperative bleeding.4,10 Embolization is used sometimes as neoadjuvant treatment in order to minimize the blood loss during surgery,6,9,19 or as only treatment, especially if surgical options are challenging and predictably associated with risk of complications.2,9 However, there are limitations that make embolization infeasible, such as the absence of vessels that can be catheterized or the vicinity to arteries supporting vital structures such as the spinal cord.2 In addition, embolization itself can be burdened by complications.2,10 Sclerotherapy, due to its capability to damage the endothelium, causing small vessel thrombosis and lesion healing, represents an alternative treatment.21,22 Radiotherapy, employed in the past alone or after surgery, is currently of limited use due to the risk of radio-induced sarcomas,10,23 vertebral body collapse, or metaphyseal fusion with growth arrest in young patients.23 Altogether, in consideration of the high risk of complications related to the traditional treatment options for ABCs, innovative therapies are needed.24
Denosumab is a human monoclonal antibody that binds the RANKL, which essentially initiates bone turnover.24,37 RANKL inhibition blocks osteoclast maturation and function.33,37,38 Denosumab has been successfully used in the treatment of osteoporosis,37,39,40 skeletal metastases,33,37,38,41 and, more recently, GCTB.37 The positive results with denosumab in the treatment of GCTB35,37 as well as the clear immunohistochemical similarity and relationship between GCTB and ABCs31,35 justify the hypothesis that denosumab may also have positive effects on ABCs.42
Although a limited number of patients have been treated to date, recent reports support this concept.5,24 Pelle et al.31 described a case of a 5-year-old boy with sacral ABC treated with denosumab, in order to avoid surgery with by a high risk of intraoperative and postoperative complications: an improvement of pain and of neurologic disease occurred after 2 and 6 weeks of treatment, respectively, with a significant reduction of tumor volume at MRI; no complications were observed. Pauli et al.5 reported a case of a 21-year-old woman with local recurrence of a proximal forearm ABC, treated with denosumab: after 5 months, the tumor was better delimitated by a bony rim, facilitating surgery. Lange et al.24 reported 2 cases of children (8 and 11 years old) treated with denosumab for spinal ABCs where embolization failed, and reported healing of the lesion after 4 months of treatment with regression of the neurologic deficits, improvement of pain, and tumor regression. We noted clinical and radiologic improvement in our series as well, with decrease of pain and paresthesia, associated with bone formation at CT scan and a decrease of uptake of gadolinium contrast on MRI. As reported in the literature, tumor volume control was seen in all patients.32 Therefore, although denosumab does not always replace surgery, it simplifies the surgical procedure by reducing tumor size when used in the neoadjuvant setting.32
Although the use of denosumab is associated with a dose-dependent risk to develop ONJ in 1.1%–2.0% of patients,34,43-46 in our series and in other reports on ABCs no complications were observed.5,24,31 Although children were not treated in our series and it is unclear if the side effect profile of denosumab differs in a preadolescent population, reports on denosumab’s successful use in children have been published.24,31 Our data support the need of prospective clinical trials to confirm the role of denosumab in ABC treatment.
Conclusions
Denosumab has substantial activity in ABCs, with favorable toxicity profile. We strongly support the use of surgery and/or embolization for the treatment of ABC, but this study clearly shows denosumab activity in the locally advanced/recurrent disease setting. Denosumab should enter the treatment armamentarium for ABC patients and could be proposed in selected patients after careful multidisciplinary discussion in a bone tumor referral center. A prospective study is warranted to confirm these findings.
Acknowledgments
The authors thank Dr. Alba Balladelli for editing the paper and Cristina Ghinelli for graphics work.
Footnotes
Conflict of interest: The authors declare they have no conflict of interest.
Funding: This work was supported by the Regione–Università Project, A. Liberati, Italian Ministry of Health–Project Alleanza contro il Cancro, and Associazione Matteo Amitrano ONLUS.
Ethical committee statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the appropriate institutional review committee.
Informed consent: Informed consent to be included in scientific studies was obtained from all individual participants included in the study at the time of admission.
References
- 1. Rapp TB, Ward JP, Alaia MJ. Aneurysmal bone cyst. J Am Acad Orthop Surg 2012; 20: 233–241. [DOI] [PubMed] [Google Scholar]
- 2. Tsagozis P, Brosjö O. Current strategies for the treatment of aneurysmal bone cysts. Orthop Rev 2015; 7: 6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mankin HJ, Hornicek FJ, Ortiz-Cruz E, et al. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol 2005; 23: 6756–6762. [DOI] [PubMed] [Google Scholar]
- 4. Cottalorda J, Chotel F, Kohler R, et al. Aneurysmal bone cysts of the pelvis in children: a multicenter study and literature review. J Pediatr Orthop 2005; 25: 471–475. [DOI] [PubMed] [Google Scholar]
- 5. Pauli C, Fuchs B, Pfirrmann C, et al. Response of an aggressive periosteal aneurysmal bone cyst (ABC) of the radius to denosumab therapy. World J Surg Oncol 2014; 12: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zenonos G, Jamil O, Governale LS, et al. Surgical treatment for primary spinal aneurysmal bone cysts: experience from Children’s Hospital Boston. J Neurosurg Pediatr 2012; 9: 305–315. [DOI] [PubMed] [Google Scholar]
- 7. Ozaki T, Halm H, Hillmann A, et al. Aneurysmal bone cysts of the spine. Arch Orthop Trauma Surg 1999; 119: 159–162. [DOI] [PubMed] [Google Scholar]
- 8. Marushima A, Matsumaru Y, Suzuki K, et al. Selective arterial embolization with n-butyl cyanoacrylate in the treatment of aneurysmal bone cyst of the thoracic vertebra: a case report. Spine 2009; 34: E230–E234. [DOI] [PubMed] [Google Scholar]
- 9. Boriani S, Lo SL, Puvanesarajah V, et al. Aneurysmal bone cysts of the spine: treatment options and considerations. Neurooncology 2014; 120: 171–178. [DOI] [PubMed] [Google Scholar]
- 10. Rossi G, Mavrogenis AF, Papagelopoulos PJ, et al. Successful treatment of aggressive aneurysmal bone cyst of the pelvis with serial embolization. Orthopedics 2012; 35: e963–e968. [DOI] [PubMed] [Google Scholar]
- 11. Lau AW, Pringle LM, Quick L, et al. TRE17/ubiquitin-specific protease 6 (USP6) oncogene translocated in aneurysmal bone cyst blocks osteoblastic maturation via an autocrine mechanism involving bone morphogenetic protein dysregulation. J Biol Chem 2010; 285: 37111–37120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oliveira AM, Chou MM, Perez-Atayde AR, Rosenberg AE. Aneurysmal bone cyst: a neoplasm driven by upregulation of the USP6 oncogene. J Clin Oncol 2006; 24: e1; author reply e2. [DOI] [PubMed] [Google Scholar]
- 13. Ye Y, Pringle LM, Lau AW, et al. TRE17/USP6 oncogene translocated in aneurysmal bone cyst induces matrix metalloproteinase production via activation of NF-kappaB. Oncogene 2010; 29: 3619–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panoutsakopoulos G, Pandis N, Kyriazoglou I, et al. Recurrent t(16;17)(q22;p13) in aneurysmal bone cysts. Genes Chromosomes Cancer 1999; 26: 265–266. [DOI] [PubMed] [Google Scholar]
- 15. Oliveira AM, Perez-Atayde AR, Inwards CY, et al. USP6 and CDH11 oncogenes identify the neoplastic cell in primary aneurysmal bone cysts and are absent in so-called secondary aneurysmal bone cysts. Am J Pathol 2004; 165: 1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Angelini A, Mavrogenis AF, Trovarelli G, Ferrari S, Picci P, Ruggieri P. Telangiectatic osteosarcoma: a review of 87 cases. J Cancer Res Clin Oncol 2016; 142: 2197–2207. [DOI] [PubMed] [Google Scholar]
- 17. Farsetti P, Tudisco C, Rosa M, et al. Aneurysmal bone cyst: long-term followup of 20 cases. Arch Orthop Trauma Surg 1990; 109: 221–223. [DOI] [PubMed] [Google Scholar]
- 18. Grzegorzewski A, Pogonowicz E, Sibinski M, et al. Treatment of benign lesions of humerus with resection and non-vascularised, autologous fibular graft. Int Orthop 2010; 34: 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pearl MS, Wolinsky JP, Gailloud P. Preoperative embolization of primary spinal aneurysmal bone cysts by direct percutaneous intralesional injection of nbutyl-2-cyanoacrylate. J Vasc Interv Radiol 2012; 23: 841–845. [DOI] [PubMed] [Google Scholar]
- 20. Shisha T, Marton-Szucs G, Dunay M, et al. The dangers of intraosseous fibro- sing agent injection in the treatment of bone cysts: the origin of major complications shown in a rabbit model. Int Orthop 2007; 31: 359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rastogi S, Varshney MK, Trikha V, et al. Treatment of aneurysmal bone cysts with percutaneous sclerotherapy using polidocanol: a review of 72 cases with long-term follow-up. J Bone Joint Surg Br 2006; 88: 1212–1216. [DOI] [PubMed] [Google Scholar]
- 22. Varshney MK, Rastogi S, Khan SA, Trikha V. Is sclerotherapy better than intralesional excision for treating aneurysmal bone cysts? Clin Orthop 2010; 468: 1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feigenberg SJ, Marcus RB, Jr, Zlotecki RA, et al. Megavoltage radiotherapy for aneurysmal bone cysts. Int J Radiat Oncol Biol Phys 2001; 49: 1243–1247. [DOI] [PubMed] [Google Scholar]
- 24. Lange T, Stehling C, Fröhlich B, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J 2013; 22: 1417–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang L, Xu J, Wood DJ, Zheng MH. Gene expression of osteoprotegerin ligand, osteoprotegerin, and receptor activator of NFkappaB in giant cell tumor of bone: possible involvement in tumor cell-induced osteoclast-like cell formation. Am J Pathol 2000; 156: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Morgan T, Atkins GJ, Trivett MK, et al. Molecular profiling of giant cell tumor of bone and the osteoclastic localization of ligand for receptor activator of nuclear factor kappaB. Am J Pathol 2005; 167: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skubitz KM, Cheng EY, Clohisy DR, et al. Gene expression in giant-cell tumors. J Lab Clin Med 2004; 144: 193–200. [DOI] [PubMed] [Google Scholar]
- 28. Bord S, Frith E, Ireland DC, et al. Synthesis of osteoprotegerin and RANKL by megakaryocytes is modulated by oestrogen. Br J Haematol 2004; 126: 244–251. [DOI] [PubMed] [Google Scholar]
- 29. Branstetter DG, Nelson SD, Manivel JC, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res 2012; 18: 4415–4424. [DOI] [PubMed] [Google Scholar]
- 30. Eghbali-Fatourechi G, Khosla S, Sanyal A, et al. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J Clin Invest 2003; 111: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pelle DW, Ringler JW, Peacock JD, et al. Targeting receptor-activator of nuclear kappaB ligand in aneurysmal bone cysts: verification of target and therapeutic response. Transl Res 2014; 164: 139–148. [DOI] [PubMed] [Google Scholar]
- 32. Dubory A, Missenard G, Domont J, Court C. Interest of denosumab for the treatment of giant-cells tumors and aneurysmal bone cysts of the spine: about nine cases. Spine 2016; 41: E654–E660. [DOI] [PubMed] [Google Scholar]
- 33. Purdue E. Aneurysmal bone cysts: denosumab extends its reach. Transl Res 2014; 164: 135–138. [DOI] [PubMed] [Google Scholar]
- 34. Henry DH, Costa L, Goldwasser F, et al. Randomized, doubleblind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol 2011; 29: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 35. Won KY, Kalil RK, Kim YW, Park YK. RANK signalling in bone lesions with osteoclast-like giant cells. Pathology 2011; 43: 318–321. [DOI] [PubMed] [Google Scholar]
- 36. Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004; 292: 490–495. [DOI] [PubMed] [Google Scholar]
- 37. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 2010; 11: 275–280. [DOI] [PubMed] [Google Scholar]
- 38. Kostenuik PJ, Nguyen HQ, McCabe J, et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res 2009; 24: 182–195. [DOI] [PubMed] [Google Scholar]
- 39. Silva-Fernández L, Rosario MP, Martínez-López JA, et al. Denosumab for the treatment of osteoporosis: A systematic literature review. Rheumatol Clin 2013; 9: 42–52. [DOI] [PubMed] [Google Scholar]
- 40. Burkiewicz JS, Scarpace SL, Bruce SP. Denosumab in osteoporosis and oncology. Ann Pharmacother 2009; 43: 1445–1455. [DOI] [PubMed] [Google Scholar]
- 41. Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012; 48: 3082–3092. [DOI] [PubMed] [Google Scholar]
- 42. Taylor RM, Kashima TG, Hemingway FK, et al. CD14– mononuclear stromal cells support (CD141) monocyte-osteoclast differentiation in aneurysmal bone cyst. Lab Invest 2012; 92: 600–605. [DOI] [PubMed] [Google Scholar]
- 43. Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010; 28: 5132–5139. [DOI] [PubMed] [Google Scholar]
- 44. Miller PD. A review of the efficacy and safety of denosumab in postmenopausal women with osteoporosis. Ther Adv Musculoskelet Dis 2011; 3: 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kyrgidis A, Toulis KA. Denosumab-related osteonecrosis of the jaws. Osteoporos Int 2011; 22: 369–370. [DOI] [PubMed] [Google Scholar]
- 46. Palmerini E, Chawla NS, Ferrari S, et al. Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Cancer 2017; 76: 118–112. [DOI] [PubMed] [Google Scholar]