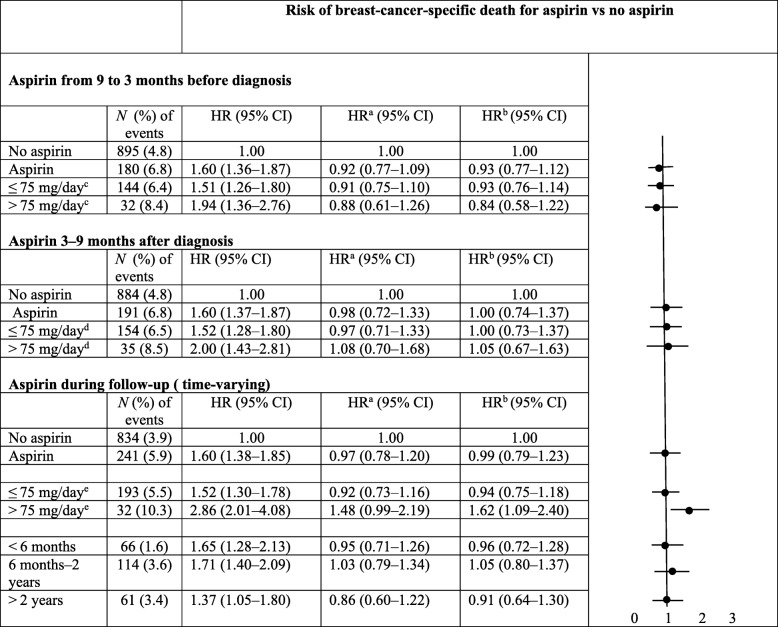
Table 2.
Aspirin use and risk for breast cancer-specific death in women with stage I–III breast cancer
CI confidence interval, HR hazard ratio
aAdjusted for age at diagnosis, stage, year of diagnosis, region, education level and comorbidity (including inflammatory diseases, heart disease, cerebrovascular disease, atherosclerotic disease, thromboembolic venous disease, hyperlipidemia, hypertension, peptic ulcer, liver disease, asthma) before diagnosis
bAdjusted for age at diagnosis, stage, year of diagnosis, region, education level, comorbidity before diagnosis, statin use, metformin use and nonsteroidal anti-inflammatory drug use (excluding aspirin) (yes/no) during the same time interval as aspirin (before or after diagnosis or during follow-up) and adjuvant oncological treatment (chemotherapy, endocrine therapy, trastuzumab and radiotherapy). In the analyses of aspirin after diagnosis and during follow-up, we additionally adjusted for prediagnostic aspirin use
c20 patients had missing information on dose
d16 patients had missing information on dose
e 258 patients (6.3% of aspirin users) changed dose during follow-up (excluded)