Abstract
Aim:
The present study was carried out to study the effect of ursolic acid (UA) as a potential anti-biofilm agent in dispersing the biofilm generated by Staphylococcus aureus isolated from milk samples of crossbred dairy cows on the day of drying. Further, in the S. aureus isolates, the presence of intracellular adherence gene locus involved in biofilm production (icaD) was investigated.
Materials and Methods:
A total of 50 S. aureus strains were isolated over a period of 3 months from 200 milk samples collected from crossbred dairy cows on the day of drying. These isolates were subjected for biofilm detection by Congo red agar (CRA), microtiter plate assay (MTP), and polymerase chain reaction specific for icaD gene. The antagonistic effect of biofilm formation by UA was studied using different concentrations (30 µg/ml and 60 µg/ml) of UA and compared with the control group.
Results:
Among the 50 S. aureus subjected for biofilm detection, 34 and 40 isolates were detected as biofilm agents by CRA and MTP methods, respectively. The in vitro studies on the effect of UA in inhibiting biofilm formation by S. aureus using MTP assay showed 71.5% and 48.6% inhibition at UA concentrations of 60 µg/ml and 30 µg/ml, respectively, with a significant difference (p<0.05) between the treated and untreated isolates, which was further evident by scanning electron microscopy. Interestingly, the isolates that were tested to be resistant through Antibiotic Sensitivity Test to commonly used antibiotics were found to be sensitive to all the tested antibiotics following UA treatment at both the tested concentrations. Furthermore, molecular detection of icaD gene for biofilm detection revealed that all the isolates that were positive by MTP had icaD gene.
Conclusion:
Increased incidence of biofilm agents in dairy infections must be considered as an alarming situation. UA treatment significantly enhanced the sensitivity of the microbial pathogens to commonly used antibiotics. Hence, attention must be paid toward implementation of new strategies such as therapeutic regimes with a combination of antibiotic and anti-biofilm agents for effective treatment of infections in dairy farms.
Keywords: biofilms, icaD, Staphylococcus aureus, ursolic acid
Introduction
Biofilm, also referred to as slime, is an extracellular polymeric conglomeration of DNA, proteins, and polysaccharides and is a significant problem in the medical, food, and marine industries often leading to substantial economic and health problems [1,2]. The microbial community of a biofilm is complex and highly resistant to antibiotics, posing a challenge of persistent infections despite antimicrobial therapies [3].
In the dairy industry, the ability of Staphylococcus species to produce biofilm is one of the primary reasons for treatment failure and recurrent infections of mammary gland [4]. Production of biofilm enables adhesion of bacteria to the epithelium of mammary glands and thus facilitates persistence in the host tissue and protection from host defense mechanisms. Production of biofilm mainly depends on the presence of the gene cluster icaADBC (the intracellular adhesion locus); strains harboring the icaADBC cluster are known to be potential biofilm producers [5]. Further, icaA and icaD were found to be in high prevalence among Staphylococcus aureus mastitis isolates implying that ica locus has a potential role as a virulence factor in the pathogenesis of mastitis in ruminants [6]. There are several conventional approaches to combating biofilms, physical and/or mechanical removal, chemical removal, and the use of antimicrobials, sanitizers, or disinfectants [4]. Recently, several compounds were tested for their efficacy in removal of biofilm generated by several microorganisms [7-19]. Ursolic acid (UA) (3-beta-3-hydroxy-urs-12-ene-28-oic-acid; UA) is a lipophilic pentacyclic triterpenoid and is widely found naturally in the peels of fruits, as well as in many herbs and spices such as lavender, oregano, thyme, rosemary, and thyme [20]. Previous studies suggested that UA from the tree Diospyros dendo added at the rate of 10 µg/ml decreased biofilm formation in Escherichia coli, Vibrio harveyi, and Pseudomonas aeruginosa by inducing chemotaxis and motility genes in bacteria [21].
The present study was carried out to study the effect of UA as a potential anti-biofilm agent in dispersing the biofilm generated by S. aureus isolated from milk samples of crossbred dairy cows on the day of drying. Further, in the S. aureus isolates, the presence of intracellular adherence gene locus involved in biofilm production (icaD) was investigated.
Materials and Methods
Ethical approval
Milk samples were collected with owners’ consent as per standard milk collection procedure. As no experimentation was performed on animals, ethical approval is not of concern to the present study.
Sample collection
A total of 200 milk samples were collected aseptically over a period of 3 months from 200 crossbred dairy cows on the day of drying from various dairy farms in and around Hyderabad, Telangana.
Bacterial isolation and phenotypic identification
Milk samples were centrifuged at 2000 g at 37°C for 10 min, supernatant was discarded, and 5 ml of brain heart infusion (BHI) broth was added to the sediment and incubated at 37°C for 24 h [22]. After incubation of milk samples in BHI broth, the morphology of the organisms was studied by Gram’s stain and cultural characters of the isolates were studied using mannitol salt agar. The isolates were also subjected to various standard biochemical tests [22,23].
Biofilm detection by Congo red agar (CRA) and Microtiter plate assay (MTP) assay
Biofilm production was evaluated by the cultivation of Staphylococcus isolates on CRA plates [24]. Isolates were interpreted according to their colony phenotypes [6]. Quantification of biofilm formation by MTP was performed according to previous studies [25,26]. The optical density (OD) of each well was measured using a microplate ELISA reader BioTek (USA) at 630 nm. Cutoff OD (ODc) is defined as three standard deviations above the mean OD of the negative control. Strains were interpreted as follows: Non-biofilm producers (OD ≤ ODc); weak biofilm producers (ODc < OD ≤2× ODc); moderate biofilm producers (2× ODc < OD ≤4× ODc); and strong biofilm producers (4× ODc < OD).
UA treatment
The antagonist effect of biofilm formation by UA was studied using different concentrations (30 µg/ml and 60 µg/ml) of UA and by its comparison with the control group that did not receive any treatment. For this, an overnight culture of biofilm-forming S. aureus was incubated for 12 h in tryptic soy broth (TSB) supplemented with 30 µg/ml and 60 µg/ml of UA (Sigma-Aldrich, CAS Number:7-55-1). Inhibitory effect was studied by MTP method as mentioned above. Anti-biofilm effect was detected by dividing the S. aureus isolates into three groups, control isolates (Group 1), UA-treated isolates at a concentration of 30 µg/ml (Group 2), and UA-treated isolates at the rate of 60 µg/ml (Group 3). The inhibitory rates were calculated using the formula:
Inhibitory rate (%) = OD (Control) - OD (Sample)/OD (Control) × 100%.
Scanning electron microscopy
Scanning electron microscopic studies were conducted for three types of samples (a) non-biofilm S. aureus, (b) biofilm-forming S. aureus, and (c) S. aureus grown in the presence of the UA (30 µg/ml). Sample preparation was done as follows: (a) Non-biofilm S. aureus: Two to three pure isolated colonies were cut from the CRA plate and placed in glutaraldehyde. (b)` Biofilm-forming S. aureus and S. aureus grown in the presence of the UA: Colony of isolates positive for biofilm was inoculated into TSB and mixed thoroughly. 1 ml of the above was dispensed into each well of a 6-well tissue culture plate. Glass coverslip (0.2 mm thick and 6 mm in diameter) was immersed into the wells. Three wells were inoculated with 30 µg/ml of UA, and the wells without UA served as a control. The plate was incubated at 37°C for 18 h in shaker incubator, and the glass coverslips were used for the microscopic study. Samples were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 24 h at 4°C followed by fixing in 2% aqueous osmium tetroxide for 4 h. These were then dehydrated in a series of graded alcohols. Processed samples were mounted over the stubs with double-sided carbon conductivity tape, and a thin layer of gold coat over the samples was done using an automated sputter coater (Model - Model JFC-1600) for 3 min. These were then scanned under scanning electron microscope (SEM - Model: JOEL-JSM5600) at required magnifications as per the standard procedures at RUSKA Labs, College of Veterinary Science, PVNRTVU, Rajendra Nagar, Hyderabad, India.
Polymerase chain reaction (PCR)
Genomic DNA isolation of bacterial isolates was carried out by phenol-chloroform DNA extraction method. The extracted DNA was dissolved in 30 µl sterile distilled water and stored in −20°C and was used for the detection of biofilm formation gene (icaD) by PCR. Primers used in PCR to amplify icaD (icaDF:5’AAACGTAAGAGAGGTGG3’ and icaDR:5’ GGCAATATGATCAAGATAC 3’) were supplied by IDT™ Bangalore with an expected amplicon size of 381bp [6]. PCR reaction mixture was set up in 25 µl consisting of DNA template 2.5 µl, Taq buffer (10×) 2.5 µl, dNTPs (10 mM) 0.5 µl, forward primer (10 µM) 2.5 µl, reverse primer (10 µM) 2.5 µl, taq polymerase 1.0 µl, and nuclease-free water 13.5 µl.
Antibiogram studies
Antibiogram was studied using antibiotic test discs manufactured by HiMedia Laboratories Limited, Mumbai and Oxoid, UK, with principle of disc diffusion method of Kirby and Bauer [22]. The antibiotics and their concentration used in the present study were ampicillin (10 µg), ceftiofur (30 µg), enrofloxacin (30 µg), gentamicin (30 µg), methicillin (30 µg), and tetracycline (30 µg). The interpretations of test were carried out according to CLSI guidelines (2013).
Statistical analysis
Data were collected and analyzed using Excel and SPSS Statistics for Windows, Version 23.0 (IBM, USA). One-way ANOVA was carried for comparing the effect of UA in inhibiting biofilm formation; p<0.05 was considered as statistically significant for univariate analysis.
Results
Bacterial isolates and identification
The isolated bacteria from dairy cows were identified by conventional methods. All of the 50 strains were found Gram-positive and catalase-positive cocci. All the 50 strains were rabbit plasma coagulase positive and were considered as S. aureus.
Biofilm detection
Among the 50 S. aureus subjected for biofilm detection, 34 and 40 isolates were detected as biofilm agents by CRA and MTP methods, respectively. The 40 isolates tested to be as biofilm producers were further tested for the presence of icaD gene and all were found to be positive for the presence of the gene (Figure-1).
Figure-1.
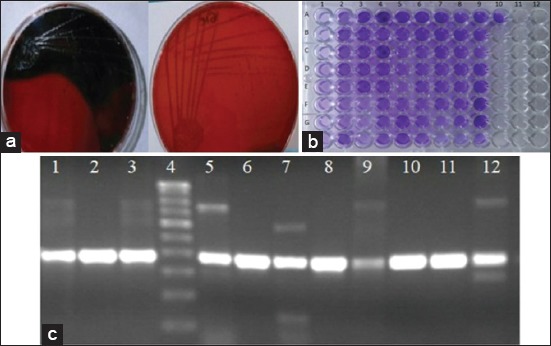
Biofilm evaluation methods of Staphylococcus aureus. (a) Differentiation of biofilm producers and non-biofilm producers on Congo red agar: Biofilm-forming S. aureus isolates produce black colonics on Congo red agar (left), as opposed to non-biofilm forming S. aureus, isolates that produce red colonies on Congo red agar (right). (b) Phenotypic characterization of biofilm formation by S. aureus isolates in microtiter plate test: Strong ability to form biofilms can be seen in wells with darker shade of blue (e.g., G9), moderate ability can be seen in wells with medium shade of blue (e.g., H5), and weak ability can be seen in wells with light shade of blue (e.g., H7). (c) (idiotypic characterization of biofilm-producing S. aureus: icaD gene was amplified from all the tested isolates (381 bp: Lane 1, 2, 3, 5, 6, 7, 8, 9, 10, 11, and 12) Lane4: 100 bp DNA Ladder.
Study of inhibitory rates (%) for biofilm formation by UA
Biofilm-forming S. aureus isolates treated with UA (30 µg/ml) showed an inhibitory rate of 48.6% when compared to that of the isolates that were untreated, as tested by MTP assay. Further, the inhibitory percentage of biofilm formation of the S. aureus isolates when treated with UA at a concentration of 60 µg/ml had increased to 71.5% (p<0.05) (Figure-2).
Figure-2.
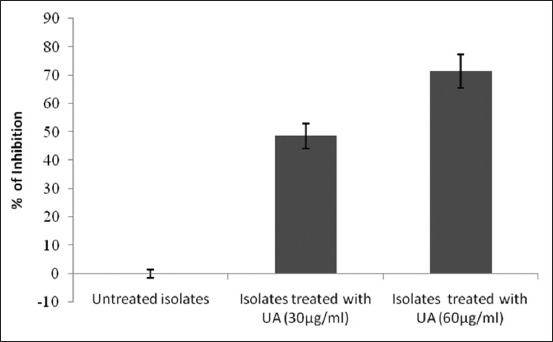
Inhibition of biofilm formation of isolates treated with U A. As tested by MTP assay, Staphylococcus aureus isolates treated with UA at concentrations of 30 pg/ml and 60 pg/ml showed an inhibitory rate of 48.6% and 71.5%, respectively, when compared with untreated isolates.
Scanning electron microscopy
Electron microscopic studies showed that non-biofilm S. aureus was in bunches with no extracellular matrix layer around the isolates. For biofilm-producing S. aureus, intracellular adhesions with a thick extracellular matrix layer were clearly seen. For isolates grown in the presence of UA, a decrease in the matrix layer was observed (Figure-3).
Figure-3.
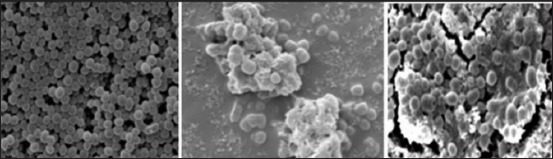
Dissolution of biofilm following UA treatment. It scanning electron microscopic image of non-biofilm Staphylococcus aureus (left). Biofilm-forming Staphylococcus aureus with intracellular adhesions and thick matrix (middle), and biofilm-forming S. aureus treated with UA showing decreased intracellular adhesions and thin matrix layer (right).
Antibiogram studies
The antibiogram pattern of S. aureus isolates treated with UA (30 µg/ml and 60 µg/ml) was compared with the untreated isolates. The results showed that hitherto-resistant isolates to various antibiotics showed increased sensitivity to the same antibiotics when treated with UA as compared to untreated isolates (Figure-4).
Figure-4.
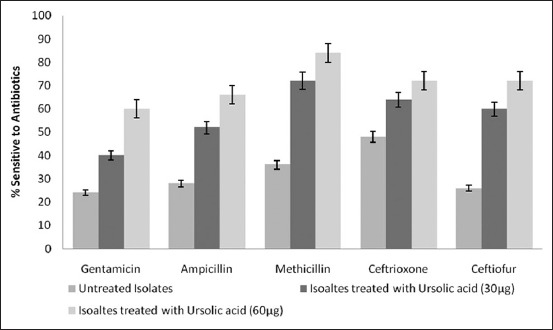
Increased sensitivity of S. aureus isolates to antibiotics following UA treatment. Increase in sensitivity to different antibiotics was noticed on the treatment of the S. aureus isolates with different concentrations of UA.
Discussion
S. aureus is a major etiological agent of bovine mastitis [27]. The losses due to mastitis are not only economical, but the side effects that ensue mastitis treatment strategies are even more alarming; major one being antimicrobial resistance due to indiscriminate use of antibiotics. Moreover, biofilm formation by certain bacterial species is known to be triggered by indiscriminate antibiotic usage [28]. Adding to it, reports of alarming increase in cases of methicillin-resistant S. aureus (MRSA) warrants immediate attention towards the control of resistant strains of S. aureus in clinical settings. Our findings revealed biofilm formation ability of mastitis pathogens, especially S. aureus isolated from milk samples on the day of drying. This suggests that efficient dry cow therapy with optimal dosage of antibiotics needs interventions in the form of anti-biofilm agents for efficient elimination and prevention of infections.
In the current study, detection of icaD gene in biofilm-forming S. aureus isolates was in accordance with findings of Samah et al. [29], who detected icaD gene in 62.5% of coagulase-positive S. aureus and 47.1% in coagulase-negative S. aureus isolated from mastitis. Considering the nature of biofilms in preventing the action of the antibiotics, we further tested the ability of UA as an anti-biofilm agent to disperse the biofilm layer around the organism and in doing so, enhanced sensitivity of the strains to the same antibiotics was also determined. UA treatment was known to inhibit biofilm formation by MRSA [30]. In the current study, UA was found to significantly disperse the biofilm around the microorganisms which could explain the enhanced sensitivity of these strains to the antibiotics which the isolates were resistant to before UA treatment. Further, to look into if there exists any relation between the biofilm production, methicillin resistance, and leukocidin toxicity; we have subjected all the 40 positive icaD gene isolates for the detection of mecA and pvl gene. Interestingly, 15 isolates of the 40 positive icaD gene isolates have shown the presence of mecA gene, and none of the isolates were positive for pvl gene (data not shown). The absence of pvl gene in the tested isolates of S. aureus indicated the absence of leukocidin toxin which could be due to the fact that the isolates in the current study were from subclinical cases and not from the clinical cases [31]. The prevalence of MRSA in the S. aureus isolates of the current study emphasizes the importance of failure in treatment strategies of bovine mastitis. Treatment strategies aimed toward a prophylactic approach of combining antimicrobials with biofilm dispersal agents can aid in combating the biofilms, thereby making the pathogenic microorganisms susceptible to antimicrobials in an efficient way [32-34].
Conclusion
Findings of the present study demonstrated a great ability of S. aureus isolates to form biofilms, even in the subclinical cases. The presence of such bacteria in the microenvironment of the udder may lead to the inefficacy of antibiotic treatment of dry cow therapy or clinical mastitis and warrants an urgent need for understanding the biofilm-forming capabilities of mastitis pathogens in designing better treatment strategies. The ability of UA as a potential antagonist of biofilms could be useful in adjunct therapies for the treatment of biofilm-involved infections and initiates the need for understanding the ability of more such compounds.
Authors’ Contributions
JSJ, KP, YNR, and KD designed the study. JSJ, KP, and MAHU performed the experiments. JSJ and KP analyzed the data and wrote the manuscript. All authors read and approved the manuscript.
Acknowledgments
The authors are thankful to the dairy farm owners (Deccan, Kapila, M.N.R, Orgaa farms, and Sri Krishna Goshala, Hyderabad) for milk sample collection. The work was funded by Department of Veterinary Microbiology, College of Veterinary Science, Rajendra Nagar. P.V.Narsimha Rao Telangana Veterinary University towards postgraduate research of J. Shiva Jyothi.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 2.Lear G, Lewis GD. Microbial Biofilms: Current Research and Applications. Wymondham: Caister Academic Press; 2012. [Google Scholar]
- 3.Sadekuzzaman M, Yang S, Mizan M.F.R, Ha SD. Current and recent advanced strategies for combating biofilms. Compr. Rev. Food Sci. Food Saf. 2015;14(5):48–66. [Google Scholar]
- 4.Melchior MB, Vaarkamp H, Fink-Gremmels J. Biofilms: A role in recurrent mastitis infections? Vet. J. 2006;171(3):398–407. doi: 10.1016/j.tvjl.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. Intracellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 1999;67(10):5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasudevan P, Nair M.K.M, Annamalai T, Venkitanarayanan KS. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol. 2003;92(1-2):179–185. doi: 10.1016/s0378-1135(02)00360-7. [DOI] [PubMed] [Google Scholar]
- 7.Haghighi F, Mohammadi SR, Mohammadi P, Hosseinkhani S, Shidpour R. Antifungal activity of TiO2 nanoparticles and EDTA on Candida albicans biofilms. Infect. Epidemiol. Med. 2013;1(1):33–38. [Google Scholar]
- 8.Packiavathy IA, Priya S, Pandian SK, Ravi AV. Inhibition of biofilm development of uropathogens by curcumin an anti-quorum sensing agent from Curcuma longa. Food Chem. 2014;148:453–460. doi: 10.1016/j.foodchem.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Molan PC. The antibacterial nature of honey. The nature of the antibacterial activity. Bee World. 1992;73(1):5–28. [Google Scholar]
- 10.Maddocks SE, Lopez MS, Rowlands RS, Cooper RA. Manuka honey inhibits the development of Streptococcus pyogenes biofilms and causes reduced expression of two fibronectin binding proteins. Microbiology. 2012;158(Pt 3):781–790. doi: 10.1099/mic.0.053959-0. [DOI] [PubMed] [Google Scholar]
- 11.Sandra SL. Essential oil show specific inhibitory effects on bacterial biofilm formation. Food Control. 2014;36(1):224–229. [Google Scholar]
- 12.Rossoni RD, Barbosa JO, Oliveira FE, Oliveira LD, Jorge AC, Junqueira JC. Biofilms of Candida albicans serotypes A and B differ in their sensitivity to photodynamic therapy. Lasers Med. Sci. 2014;29(5):1679–1684. doi: 10.1007/s10103-014-1570-z. [DOI] [PubMed] [Google Scholar]
- 13.Padmavathi AR, Pandian SK. Antibiofilm activity of biosurfactant producing coral associated bacteria isolated from Gulf of Mannar. Indian J. Microbiol. 2014;54(4):376–382. doi: 10.1007/s12088-014-0474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambanthamoorthy K, Feng X, Patel R, Patel S, Paranavitana C. Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiol. 2014;14(1):197. doi: 10.1186/1471-2180-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pradhan AK, Pradhan N, Sukla LB, Panda PK, Mishra BK. Inhibition of pathogenic bacterial biofilm by biosurfactant produced by Lysinibacillus fusiformis S9. Bioprocess Biosyst. Eng. 2014;37(2):139–149. doi: 10.1007/s00449-013-0976-5. [DOI] [PubMed] [Google Scholar]
- 16.Wu ZY, Ye CS, Guo F, Zhang SH, Yu X. Evidence for broad-spectrum biofilm inhibition by the bacterium Bacillus spp strain SW9. Appl. Environ. Microbiol. 2013;79(5):1735–1738. doi: 10.1128/AEM.02796-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pradhan AK, Pradhan N, Mall G, Panda HT, Sukla LB, Panda PK, Mishra BK. Application of lipopeptide biosurfactant isolated from a halophile Bacillus tequilensis CH for inhibition of biofilm. Appl. Biochem. Biotechnol. 2013;171(47):1362–1375. doi: 10.1007/s12010-013-0428-3. [DOI] [PubMed] [Google Scholar]
- 18.Yamakami K, Tsumori H, Sakurai Y, Shimizu Y, Nagatoshi K, Sonomoto K. Sustainable inhibition efficacy of liposome-encapsulated nisin on insoluble glucan-biofilm synthesis by Streptococcus mutans. Pharm. Biol. 2013;51(2):267–270. doi: 10.3109/13880209.2012.717227. [DOI] [PubMed] [Google Scholar]
- 19.Marvasi M, Carrazana M, Durie I, Teplitski M. Systemic analysis of the ability of nitric oxide donors to dislodge biofilms formed by Salmonella enterica and Escherichia coli O157: H7. AMB Express. 2014;4(1):42. doi: 10.1186/s13568-014-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wozniak T, Skąpska S, Marszatek K. Ursolic acid - A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules. 2015;20(11):20614–20641. doi: 10.3390/molecules201119721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren D, Zuo R, Gonz´alez-Barrios AF, Bedzyk LA, Eldridge GR, Pasmore ME, Wood TK. Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl. Environ. Microbiol. 2005;71(7):4022–4034. doi: 10.1128/AEM.71.7.4022-4034.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruickshank R, Duguid JP, Marmion BP, Swain RHA. Medical Microbiology. 12th ed. Edinburgh: Churchill Livingstone; 1975. [Google Scholar]
- 23.Patricia T. Bailey and Scott's Diagnostic Microbiology. 14th ed. Netherlands: Mosby, Elsevier; 2012. [Google Scholar]
- 24.Mathur S.T.S, Khan S, Upadhyay DJ, Fatma T, Rattan A. Detection of biofilm formation among the clinical isolates of staphylococci: An evaluation of three different screening methods. Indian J. Med. Microbiol. 2006;24(1):25–29. doi: 10.4103/0255-0857.19890. [DOI] [PubMed] [Google Scholar]
- 25.Dubravka M, Lazis VB, Petrovi J, Bugarski D, Eguljev Z. Slime production and biofilm-forming ability by Staphylococcus aureus bovine mastitis isolates. Acta Vet. 2010;60(2-3):217–226. [Google Scholar]
- 26.Stepanovic S, Dakic I, Opavski N, Jezek P, Ranin L. Influence of the growth medium composition on biofilm formation by Staphylococcus sciuri. Ann. Microbiol. 2003;53(1):63–74. [Google Scholar]
- 27.Lasa I, Penades J. Bap: A family of surface proteins involved in biofilm foration. Res. Microbiol. 2006;157(2):99–107. doi: 10.1016/j.resmic.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2004;48(7):2633–2636. doi: 10.1128/AAC.48.7.2633-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samah F, Darwish A, Hanaa AE. Investigation of biofilm-forming ability in staphylococcicausing bovine mastitis using phenotypic and genotypic assays. Sci. World J. 2013;2013(37):9. doi: 10.1155/2013/378492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nan Q, Tan X, Jiao Y, Liu L, Zhao W, Yang S, Jia A. RNA-seq-based transcriptome analysis of Methicillin-resistant Staphylococcus aureus biofilm inhibition by ursolic acid and resveratrol. Sci. Rep. 2014;4:5467. doi: 10.1038/srep05467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awad A, Ramadan H, Nasr S, Ateya A, Atwa S. Genetic characterization, antimicrobial resistance patterns and virulence determinants of Staphylococcus aureus isolated from bovine mastitis. Pak. J. Biol. Sci. 2017;20(6):298–305. doi: 10.3923/pjbs.2017.298.305. [DOI] [PubMed] [Google Scholar]
- 32.Stewart PS. Prospects for anti-biofilm pharmaceuticals. Pharmaceuticals. 2015;8(3):504–511. doi: 10.3390/ph8030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan JB. Therapeutic potential of biofilm-dispersing enzymes. Int. J. Artif. Organs. 2009;32(9):533–695. doi: 10.1177/039139880903200903. [DOI] [PubMed] [Google Scholar]
- 34.Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 2008;74(2):470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


