Figure 7: DSPE-PEG peptides can be presented by human DCs, with antigen processing similar to unmodified peptides.
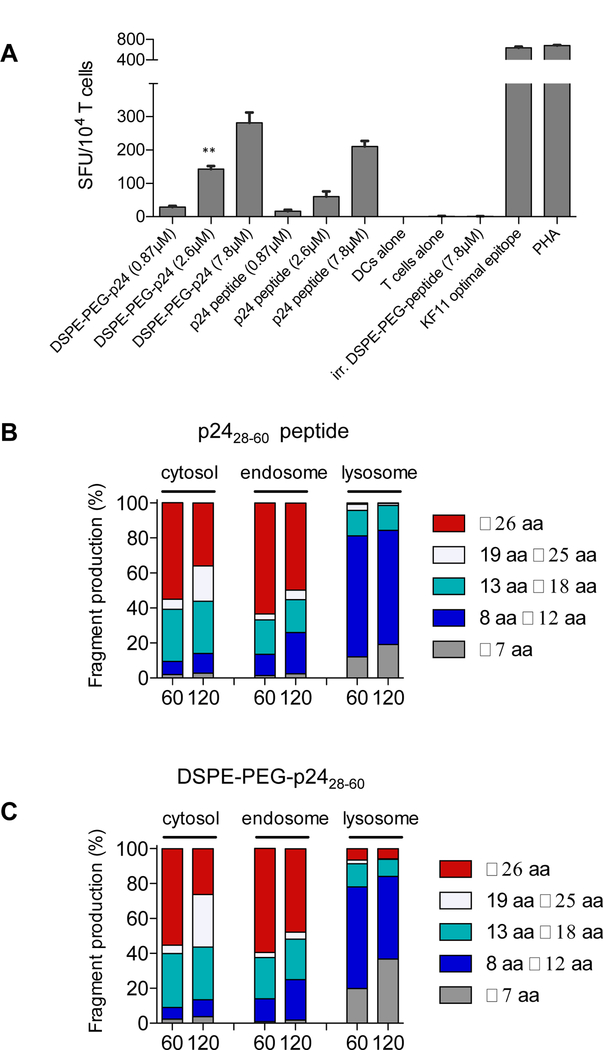
A, Monocyte-derived DCs were generated from HLA-B57+ PBMCs and incubated with free HIV p2428–60 peptide or DSPE-PEG-p2428–60 for 4 hours, washed, and plated overnight with KF11 CD8+ T-cells in a 2:1 effector-to-target ratio. Spot forming units (SFU) per 104 KF11+ T cells quntified. n = 3/group; student’s t-test for indicated comparisons between DSPE-PEG-p2428–60 and p2428–60 peptide for a given concentration. B-C, p2428–60 peptide or DSPE-PEG-p2428–60 (2 nmol) were incubated with cell extracts from endosomal, lysosomal, or cytosolic cellular compartments isolated from human APCs. Resulting peptides were purified and analyzed via mass spectrometry. Shown are the lengths of peptide fragments produced under cytosolic, endosomal, or lysosomal degradation conditions for 60 or 120 minutes. *P<0.05, **P<0.01, ***P<0.001.
