Abstract
Evidence suggests that sex differences in response to cocaine administration may be regulated by activation of progesterone and estrogen receptors. To test this hypothesis, rats were pretreated with either RU 486 (progesterone antagonist; 0, 3, or 25 mg/kg), tamoxifen (estrogen antagonist; 0, 1, or 3 mg/kg), or vehicle followed by saline or cocaine administration (15 mg/kg). Although RU 486 did not affect cocaine-induced locomotor activity in female rats, it dose-dependently decreased such activity in males (3 mg/kg significantly attenuated locomotor responses in cocaine-treated rats as compared with vehicle treatment or 25 mg/kg of RU 486). RU 486 also affected baseline serum levels of corticosterone. Males treated with 3 mg/kg of RU 486 plus cocaine had higher progesterone and corticosterone serum levels than vehicle-treated groups. In females, both doses (3 and 25 mg/kg) of RU 486 significantly attenuated corticosterone serum levels compared with vehicle treatment. For both sexes overall, tamoxifen neither significantly influenced cocaine-induced ambulatory and rearing responses nor altered cocaine-induced progesterone and corticosterone serum levels. Taken together, our results suggest that progesterone receptors have a sexually dimorphic role in cocaine-induced effects, but estrogen receptors have only a limited role. Moreover, both receptor antagonists modulate neuro-chemical responses differentially.
Keywords: Cocaine, Progesterone Receptor, Estrogen Receptor, Sex Differences
Introduction
Accumulating evidence suggests sex differences in response to cocaine administration. For example, women report more drug cravings, use greater amounts of drugs, and are admitted to the emergency department more frequently than men.1–3 Similarly, female rats acquire cocaine discrimination at a faster rate, display a greater motivation to self-administer cocaine, and exhibit more augmented behavioral responses to cocaine than do male rats.4 Female rats also develop conditioned place preference to cocaine with lower doses and fewer conditioning days than male rats require.5 These results suggest that females are more sensitive than males to the addictive properties of cocaine.
Through different experimental approaches, it has been shown that gonadal hormones may contribute in part to sex differences in behavioral and endocrinologic responses to cocaine. For example, rodent studies have shown that hormonal fluctuations during the female reproductive cycle modulate cocaine-induced behavioral responses; higher locomotor activities have been shown during estrus and proestrus than diestrus.6–8 Gonadectomy of female rats decreases overall cocaine-induced loco-motor responses, but in male rats the effects of gonadectomy are inconsistent.9–12 In female rats, gonadal hormone replacement affects cocaine-induced locomotor response, ie, estrogen increases cocaine-induced locomotor responses, whereas progesterone attenuates them.7,13–15 However, the mechanisms by which estrogen and progesterone influence cocaine-stimulated responses are not well understood.
RU 486 (mifepristone), a progesterone receptor antagonist, and tamoxifen, a selective estrogen-receptor modulator, are commonly used to study the role of steroid receptors in the modulation of behavioral activities since both compounds inhibit lordosis behaviors.16–18 In this study, these pharmacologic agents were used to test the hypothesis that activation of progesterone or estrogen receptors is a necessary step in mediating some behavioral effects of gonadal hormones on cocaine-induced activity in both male and female rats.
Methods
Animals
Eight-week-old intact male and female Fischer rats purchased from Charles River (Raleigh, NC) were individually housed in standard cages with free access to standard chow and water ad libitum. Rats were maintained on a 12-hour light/dark cycle (lights on at 9 AM) and handled for 1 week before experimental manipulations. RU 486 and tamoxifen studies were run separately. Each study consisted of three cohorts, run 1 month apart, with 8–12 rats per group. Because vaginal lavages cause behavioral and neurochemical changes that may account for the differences observed between female and male rats, females were randomly assigned to experimental groups regardless of their estrous cycles.8 All National Institutes of Health guidelines for the care and use of laboratory animals were strictly followed and were approved by the Institutional Animal Care and Use Committee of Hunter College.
Drugs
Rats were pretreated for 1 hour with RU 486 (0, 3, or 25 mg/kg) or tamoxifen (0, 1, or 3 mg/kg) followed by intraperitoneal injections of saline or cocaine (15 mg/kg dissolved in .9% saline). Two different control groups were run to offset differences in vehicle-treatment and manner of administration (dimethyl sulfoxide [DMSO, 100%] and intraperitoneal injection for the RU 486 cohort; sesame oil and subcutaneous injection for the tamoxifen cohort). The cocaine dose was previously reported to effectively induce behavioral and hormonal responses in both male and female rats without producing a maximal effect.19,20 The RU 486 and tamoxifen doses were chosen because they decrease lordosis and nociceptive behavioral responses in female rats.16,21,22
Behavioral Activity
All behavioral activities were recorded by using a spontaneous locomotor activity (San Diego Instrument, San Diego, Calif). Ambulatory and rearing responses were recorded for 30 minutes after drug treatment in the rat’s home cage, as previously described.9 Ambulatory activity represents the number of counts produced by two consecutive photobeam interruptions in the lower frame. Rearing activity represents total counts of vertical motion as recorded in the upper frame.
Serum levels of Progesterone and Corticosterone
After a brief exposure to CO2 (20 seconds), rats were decapitated, and serum was collected after centrifuging the trunk blood at 3000 rpm for 30 minutes. Samples (from the same rats used for the behavioral analysis) were analyzed for progesterone and corticosterone by using Coat-A-Count radioimmunoassay kits from Diagnostic Products (Los Angeles, Calif). Intra-assay coefficients of variation were <10.0%±1.0%. Hormone levels were determined by a log-logit analysis using GraphPad Prism (GraphPad Software, Inc., San Diego, Calif). Serum levels of progesterone and corticosterone were expressed in nanograms per milliliter.
Statistical Analysis
Data on locomotive activity and hormone levels were presented as the average of each treatment group plus or minus standard error of the mean. For each behavioral measurement, two-way analyses of variance were conducted to determine within the sexes the effects of cocaine and the antagonist’s pretreatment (drug [cocaine or saline] × antagonist dose) or to determine, by sex, the effects of cocaine and behavioral responses (drug [cocaine or saline] × sex [male vs female]). When appropriate, Fisher least significant difference post-hoc tests were used. A P value <.05 was considered significant in all statistical analyses.
Results
Sex Differences in Cocaine-Induced Behavioral Activity
In both male and female rats, cocaine increased all behavioral activities (ambulation: F[1,28]556.00, P<.05; rearing: F[1,28]5150.91, P<.05; Figure 1). A main effect of drug and sex was observed in ambulatory and rearing counts (ambulation: F[1,28]515.42, P<.01; rearing: F[1,28]=16.08, P<.05); cocaine-treated female rats ambulated and reared significantly more than did cocaine-treated male rats (P<.01).
Fig 1.
Effects of cocaine on ambulatory (A) and rearing counts (B) in male and female rats. Data are represented as cumulative ambulatory and rearing counts for the 30 minutes of behavioral testing. *Significant differences between saline- and cocaine-treated rats. #Significant differences between male and female treatment groups (P<.05).
Effects of RU 486 on Cocaine-Induced Behavioral Activation in Male and Female Rats
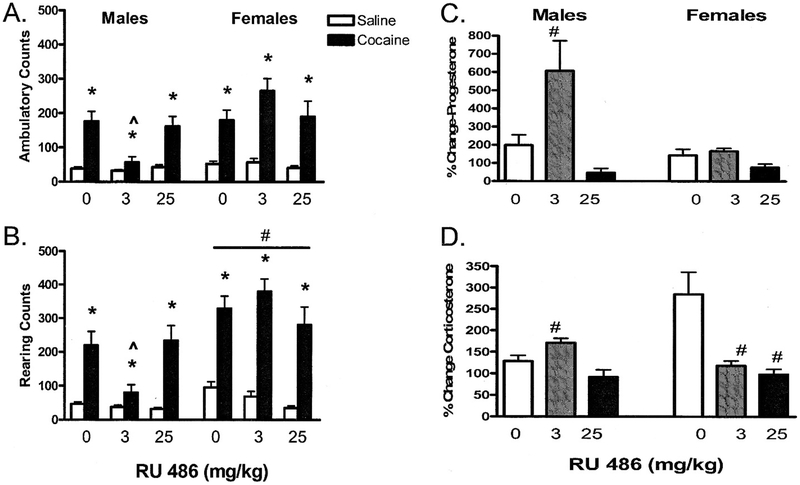
As shown in Figure 2, significant interactions between drug and RU 486 treatments were observed in males for ambulatory and rearing activities (ambulation: F[2,74]=4.22, P=.02; rearing: F[2,74]=3.42, P=.04; Figure 2A and B); 3 mg/kg of RU 486 significantly attenuated ambulatory and rearing activities in cocaine-treated male rats when compared with vehicle- or 25 mg/kg-treated rats (P<.05 for all comparisons). In female rats, no significant differences in cocaine-induced ambulatory or rearing counts were observed between vehicle and any of the RU 486 pretreatments.
Fig 2.
Dose effect of RU 486 on cocaine-induced ambulatory counts (A), rearing counts (B), progesterone serum levels (C), and corticosterone serum levels (D) in male and female rats. For A and B, data are represented as cumulative ambulatory and rearing counts for the behavioral testing. *Significant differences between saline- and cocaine-treated rats. ^Significant main effect of RU 486 dose.^^Significant interaction effect of cocaine and RU 486 dose. For C and D, normalized progesterone and corticosterone serum levels for cocaine-treated rats. #Significant difference between vehicle- and antagonist-treated group (P<.05).
Effects of RU 486 on Cocaine-Induced Changes in Serum Levels of Progesteroneand Corticosterone
In female rats, RU 486 administration raised serum levels of corticosterone in saline-treated controls (F[2,28]=10.89, P<.05; Table); saline-treated rats receiving either 3 mg/kg or 25 mg/kg of RU 486 had higher serum levels of corticosterone than those who received no RU 486 (P<.05). Similarly, RU 486 altered serum levels of progesterone in saline-treated male and female rats (P<.05). Thus, to account for these baseline effects when analyzing for cocaine responses, progesterone and corticosterone data were normalized to percentage of saline (Figure 2C).
In both male and female rats, significant effects of drug and antagonist interaction on serum levels of corticosterone were observed (F[2,31]=7.95, P<.05; F[2,32]=10.06, P<.05, Figure 2D). In male rats, 3 mg/kg of RU 486 significantly increased cocaine-induced corticosterone serum levels (P=.04). However, in female rats, both 3 mg/kg and 25 mg/kg of RU 486 significantly attenuated corticosterone serum levels as compared with their respective vehicle controls (P<.05). In male rats, RU 486 dose-dependently affected cocaine-induced alterations in serum levels of progesterone (P<.05); 3 mg/kg of RU 486 increased progesterone serum levels by sixfold (P<.001).
Effects of Tamoxifen on Cocaine-Induced Behavioral Response and Serum Levels of Progesterone and Corticosterone
As illustrated in Figure 3, a main effect of drug was observed in cocaine-treated male and female rats; in both sexes, cocaine-treated rats had higher ambulatory and rearing counts than saline-treated groups (males: ambulatory counts: F[1,61]=14.53, P<.05; rearing counts: F[1,62]=22.18, P<.05; females: ambulatory counts: F[1,66]=48.60, P<.05; rearing counts: F[1,68]=68.76, P<.05; Figure 3 A and B). However, tamoxifen did not significantly alter cocaine-induced behavioral responses in either sex. Nor did tamoxifen significantly change cocaine-induced effects on serum levels of either progesterone or corticosterone (Figure 3 C and D).
Fig 3.
Dose effect of tamoxifen on cocaine-induced ambulatory counts (A), rearing counts (B), progesterone serum levels (C), and corticosterone serum levels (D) in male and female rats. In A and B, rats were pretreated for 1 hour with tamoxifen (1 or 3 mg/kg) followed by 30 minutes of saline (white bars) or cocaine (solid bars) administration. *Significant differences between saline- and cocaine-treated rats. C and D show normalized progesterone and corticosterone serum levels. #Significant difference between vehicle- and antagonist-treated group (P<.05).
Discussion
Similar to the results of Chin et al23 and Festa et al,20 cocaine-treated female rats in this study displayed greater rearing and ambulatory responses than did cocaine-treated male rats. However, no sexually dimorphic responses were observed in ambulatory activities after cocaine and RU 486 coadministration. This outcome may be partly due to the DMSO pretreatment in control groups, which has previously been shown to affect behavioral activities.24 Indeed, sex differences in ambulatory activity were observed in vehicle- and saline-treated rats in the tamoxifen study, further supporting the hypothesis that DMSO abolished the known sex differences in ambulatory responses.
In male rats, the attenuation of cocaine-induced ambulatory and rearing behavioral responses after pretreatment with lower doses of RU 486 suggests that activation of progesterone receptors may be a necessary step in mediating some of those responses. RU 486’s binding affinity for the progesterone receptors is dose dependent; lower doses of RU 486 are potent antiprogestins, whereas higher doses have both anti-glucocorticoid and antiprogestin activi ties.25 The dose-dependent effects of RU 486 on the inhibition of ambulatory and rearing responses to cocaine may be partly explained by this pharmacologic effect. Because female rats have higher intrinsic concentrations of progesterone receptors in areas important for cocaine-induced motor activation (unpublished observations), the RU 486 doses used here may have been unable to completely block progesterone receptors in the mesocorticolimbic areas and thus failed to alter the females’ responses to cocaine.
Consistent with previous findings in humans,26 RU 486 dose-dependently increased corticosterone serum levels in control groups of both male and female rats. Pretreatment with DMSO also increases corticosterone serum levels.27 Moreover, the fact that in the vehicle-treated groups of the tamoxifen study no baseline effects on corticosterone serum levels were observed further confirms that RU 486 or DMSO may alter corticosterone serum levels.
RU 486 affected cocaine-induced corticosterone and progesterone responses in a dose-dependent and sexually dimorphic manner. In male rats, 3 mg/kg RU 486 increased cocaine-stimulated corticosterone and progesterone serum levels; in female rats, both 3 mg/kg and 25 mg/kg RU 486 significantly attenuated cocaine’s effects on corticosterone levels. However, the mechanism by which sex alters the direction of these effects has yet to be determined. Because females have higher corticosterone and progesterone serum levels than males and both hormones are drastically induced in females after cocaine administration as compared with males,9,19,23 the pharmacokinetics of RU 486 in females may differ from that in males. However, because of the strong effect of the solvent DMSO on the hypothalamic-pituitary-adrenal axis and the possible indirect effect on progesterone serum levels, caution must be exercised when interpreting our observations. Interestingly, 3 mg/kg RU 486 attenuated cocaine-induced ambulatory and rearing responses as well as increased progesterone serum levels by 10-fold in male rats. Thus, on the basis of previous observations that demonstrated that progesterone attenuates cocaine-induced locomotor and rewarding responses,7,13–15 RU 486 attenuation of cocaine-induced behavioral responses may in part be mediated through this induction of progesterone serum levels.
Tamoxifen neither altered cocaine-induced ambulatory and rearing activities nor had an effect on corticosterone or progesterone serum levels. Thus, estrogen receptor activation may play a limited role in behavioral and neuroendocrino-logic responses to acute cocaine administration. Indeed, estrogen replacement consistently has been shown to have no effect on cocaine-induced behavioral responses after acute administration.4 However, chronic estrogen replacement enhances cocaine-induced behavioral responses regardless of concentration or replacement paradigm.10,15,28,29 This, in turn, suggests that estrogen’s effects may pertain only to behavioral responses after chronic cocaine administration. Taken together, our results suggest that the role of progesterone receptor activation in cocaine-induced responses is sexually dimorphic, but estrogen receptor activation plays only a limited role in behavioral responses to acute cocaine administration in both male and female rats.
Table 1.
Corticosterone and progesterone serum levels after RU 486 or tamoxifen and cocaine treatments
| Concentration | Drug | Male | Female | ||
|---|---|---|---|---|---|
| RU 486 | Corticosterone | 0 mg/kg | Saline | 417.40±670.5 | 185.8±33.7# |
| Cocaine | 536.4±54.2 | 528.5±97.4 | |||
| 3 mg/kg | Saline | 407.6±68.1 | 737.2±129.7#^ | ||
| Cocaine | 644.8±65.1 | 868.7±83.8 | |||
| 25 mg/kg | Saline | 558.8±88.0 | 848.0±126.2#^ | ||
| Cocaine | 510.2±97.1 | 830.2±104.1 | |||
| Progesterone | 0 mg/kg | Saline | 4.3±1.7^ | 14.9±3.4 | |
| Cocaine | 8.6±2.3* | 21.3±4.8 | |||
| 3 mg/kg | Saline | 2.8±1.1 | 21.8±7.0^ | ||
| Cocaine | 16.7±4.2 | 36.3±3.2 | |||
| 25 mg/kg | Saline | 10.9±3.7 | 32.7±3.0^ | ||
| Cocaine | 5.1±2.4 | 25.5±5.5 | |||
| Tamoxifen | Corticosterone | 0 mg/kg | Saline | 117.7±33.5 | 74.4±13.2^ |
| Cocaine | 241.2±88.9* | 227.4±46.5* | |||
| 1 mg/kg | Saline | 124.4±51.5 | 68.5±22.6^ | ||
| Cocaine | 173.5±53.1* | 139.9±28.3* | |||
| 3 mg/kg | Saline | 82.2±8.5 | 156.7±38.1^ | ||
| Cocaine | 103.9±37.3* | 634.0±103.4* | |||
| Progesterone | 0 mg/kg | Saline | .9±1.7^ | 12.5±3.2 | |
| Cocaine | .6±3.6 | 15.3±3.3 | |||
| 1 mg/kg | Saline | .3±.1^ | 8.3±1.9 | ||
| Cocaine | .2±.1 | 11.2±2.3* | |||
| 3 mg/kg | Saline | .3±.1^ | 7.5±1.8 | ||
| Cocaine | .3±.1 | 12.7±1.7* |
Data are presented as mean ± standard error of the mean of corticosterone and progesterone serum levels (ng/mL).
Significant main effect of saline-treated controls.
Significant main effect of RU 486 or tamoxifen doses from saline-treated groups.
Significant differences between saline- and cocaine-treated rats.
Acknowledgments
This work was supported by SNRP NF 39534, RR 03037, DA12136, and 506-GM 60654.
References
- 1.Dudish SA, Pentel PR, Hatsukami DK. Smoked cocaine self-administration in females. Psychopharmacology. 1996;15:392–405. [DOI] [PubMed] [Google Scholar]
- 2.Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–66. [DOI] [PubMed] [Google Scholar]
- 3.Robbins SJ, Ehrman RN, Chidress AR, O’Brian CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. [DOI] [PubMed] [Google Scholar]
- 4.Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. [DOI] [PubMed] [Google Scholar]
- 5.Russo SJ, Festa ED, Fabian SJ, et al. Gonadal hormones differentially modulate cocaine- induced place preference in male and female rats. Neuroscience. 2003;120:523–533. [DOI] [PubMed] [Google Scholar]
- 6.Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ. Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res. 1999;101:15–20. [DOI] [PubMed] [Google Scholar]
- 7.Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J Pharmacol Exp Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- 8.Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. [DOI] [PubMed] [Google Scholar]
- 9.Chin J, Sternin O, Wu HBK, et al. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002;945:123–130. [DOI] [PubMed] [Google Scholar]
- 10.Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–927. [DOI] [PubMed] [Google Scholar]
- 12.Walker QD, Cabassa J, Hilmar AS, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25: 118–130. [DOI] [PubMed] [Google Scholar]
- 13.Peris J, Decambre N, Coleman-Hardee ML, Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal (3H) dopamine release. Brain Res. 1991;566:255–264. [DOI] [PubMed] [Google Scholar]
- 14.Perrotti LI, Lu D, Niyomachai T, et al. Temporal effects of estrogen and progesterone on behavioral and endocrinological responses to acute cocaine administration. Cell Mol Biol. 2003;49:1269–1274. [PubMed] [Google Scholar]
- 15.Perrotti LI, Russo SJ, Fletcher H, et al. Ovarian hormones modulate cocaine induced locomotor and stereotypic activity. Ann N Y Acad Sci. 2001;937:202–216. [DOI] [PubMed] [Google Scholar]
- 16.Brown TJ, Moore MJ, Blaustein JD. Maintenance of progesterone-facilitated sexual behavior in female rats requires continued hypothalamic protein synthesis and nuclear progestin receptor occupation. Endocrinology. 1987;121: 298–304. [DOI] [PubMed] [Google Scholar]
- 17.Etgen AM, Shamamian P. Regulation of estrogen-stimulated lordosis behavior and hypothalamic progestin receptor induction by antiestrogens in female rats. Horm Behav. 1986;20:166–180. [DOI] [PubMed] [Google Scholar]
- 18.Patisaul HB, Luskin JR, Wilson ME. A soy supplement and tamoxifen inhibit sexual behavior in female rats. Horm Behav. 2004;45:270–277. [DOI] [PubMed] [Google Scholar]
- 19.Festa ED, Jenab S, Chin J, et al. Frequency of cocaine administration affects behavioral and endocrine responses in male and female Fischer rats. Cell Mol Biol. 2003;49:1275–1280. [PubMed] [Google Scholar]
- 20.Festa ED, Russo SJ, Gazi FM, et al. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–687. [DOI] [PubMed] [Google Scholar]
- 21.Apostolakis ME, Garai J, Clark JH, O’Malley BW. In vivo regulation of central nervous system progesterone receptors: cocaine induces steroid-dependent behavior through dopamine transporter modulation of D5 receptors in rats. Mol Endocrinol. 1996;10:1595–1604. [DOI] [PubMed] [Google Scholar]
- 22.Etgen AM. Antiestrogens: effects of tamoxifen, nafoxidine, and CI-628 on sexual behavior, cytoplasmic receptors, and nuclear binding of estrogen. Horm Behav. 1979;13:97–112. [DOI] [PubMed] [Google Scholar]
- 23.Chin J, Sternin O, Wu HBK, et al. Sex differences in cocaine-induced behavioral sensitization. Cell Mol Biol. 2001;47:1089–1095. [PubMed] [Google Scholar]
- 24.Izzo E, Martin-Fardon R, Koob GF, Weiss F, Sanna PP. Neural plasticity and addiction: PI3-kinase and cocaine behavioral sensitization. Nat Neurosci. 2002;5:1263–1264. [DOI] [PubMed] [Google Scholar]
- 25.Gaillard RC, Riondel A, Muller AF, HermannW. RU486: a steroid with antiglucocorticoid steroid activity that only disinhibits the human pituitary adrenal system at a specific time of day. Proc Natl Acad Sci U S A. 1984;81:3879–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bamberger CM, Chrousos GP. The glucocorticoid receptor and RU 486 in man. Ann N Y Acad Sci. 1995;761:296–310. [DOI] [PubMed] [Google Scholar]
- 27.Matic SI, Dinic S, Mihailovic M, Grigorov I, Bogojevic D, Poznanovic G. Acute-phase protein expression in DMSO-intoxicated rats. Toxicol Lett. 2004;147:153–159. [DOI] [PubMed] [Google Scholar]
- 28.Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–290. [DOI] [PubMed] [Google Scholar]
- 29.Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. J Pharmacol Exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]