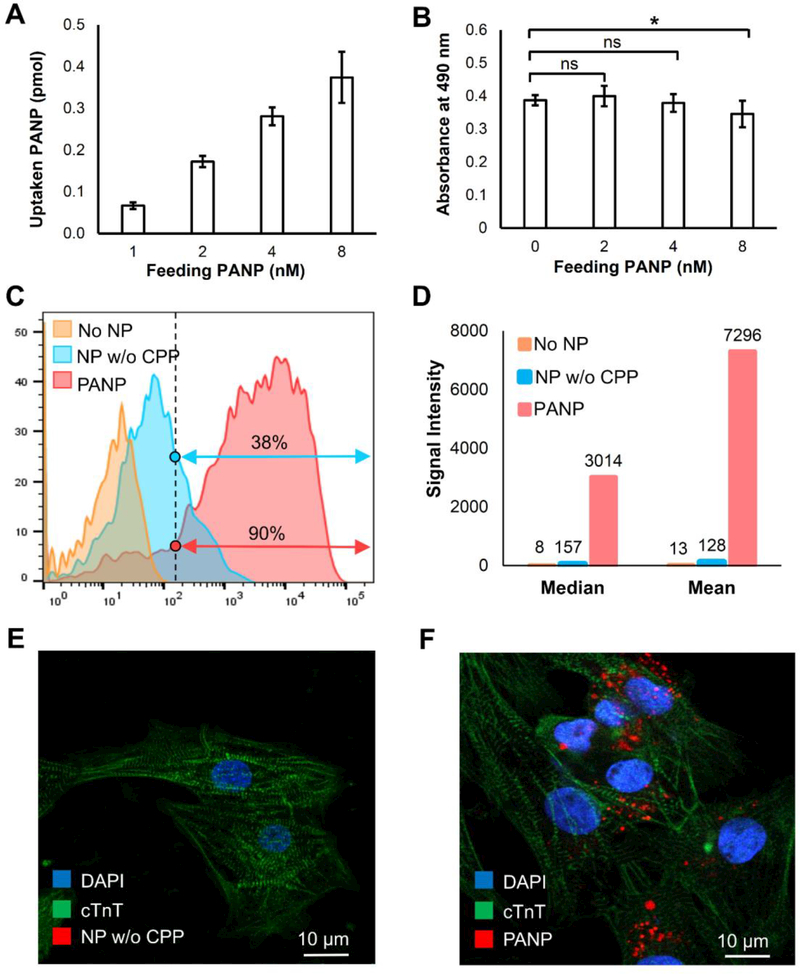
Figure 2. Assessing the uptake, toxicity, and efficiency of hESC-CM labeling with PANPs in vitro.
We first compared the uptake ratio and toxicity of hESC-CM labeling under different feeding PANP doses. (A) A higher feeding PANP dose from 1 nM to 8 nM led to a higher uptake rate for cells. (B) The PANPs showed significant toxicity to the labeled hESC-CMs when the feeding dose of PANPs exceeded 4 nM. Using feeding PANP dose of 4 nM, we further compared two approaches for direct labeling of hESC-CMs: our proposed PANP-based labeling versus the routine endocytosis-based labeling that used the nanoparticles without CPPs (NP w/o CPP). (C) Following an overnight labeling, FACS-based quantification indicated that the percentage of hESC-CMs labeled by PANPs was 2.4 times more than that labeled by NPs w/o CPP. (D) The fluorescence intensity analysis further indicated that the loaded dose of PA contrast agents with PANP labeling was almost 60 times higher than that labeled by NPs w/o CPP. (E-F) Confocal images of delivered NP w/o CPP and PANP (red) in hESC-CMs, which revealed no fluorescence from NP w/o CPP in cells (left) due to the ultra-low internalization efficiency through endocytosis.