Abstract
Background
Induction immunosuppression is used in transplantation to prevent early acute rejection. The survival benefit of rabbit anti-thymocyte globulin (rATG) induction has not been established yet. We sought to determine the role of rATG in preventing rejection and improving overall survival.
Material/Methods
A retrospective cohort study was conducted from 2005 to 2009 and data of consecutive 268 heart transplant recipients were reviewed.
Results
The data of 144 patients who received induction with rATG were compared to 124 patients who did not. Although overall survival was not different between the 2 groups (P=0.12), there was a significant difference in restricted mean survival time (RMST) at 5 years (RMST=4.8 months; 95% CI: 1.0–8.6, P=0.01) and 10 years (RMST=10.4 months; 95% CI: 1.6–19.3, P=0.02) in favor of the non-induced patients. No difference was observed between induced and non-induced patients who developed de novo donor specific antibodies. There was a significant difference in median days to first rejection in favor of the induced group (P<0.001).
Conclusions
Induction with rATG adds no survival benefit in heart transplant recipients. Patients who did not receive induction therapy had higher life expectancy at 5 years and 10 years. Although there was significant delay in the first rejection episode in favor of the rATG induced group, no difference was observed in donor specific antibodies. This study indicates a need for separate analysis of peri-transplantation co-morbidities and mainly the incidence of acute kidney injury, which could affect long-term survival.
MeSH Keywords: Graft Rejection, Heart Transplantation, Survival
Background
The immunosuppressive regimens that could be used for organ transplantation include induction, maintenance, or rescue therapies. Induction immunosuppression is used at the time of transplantation based on the observation that more intense immunosuppression is required to prevent early acute rejection [1,2]. Approximately 20% of all adult heart transplant patients in the USA, 30% of patients in Europe [3], and approximately half of all pediatric recipients [4] receive rabbit anti-thymocyte globulin (rATG) induction, but its use varies widely between countries and centers. Although rATG has been licensed for 30 years, there is still a lack of well-conducted trials examining its efficacy and safety as an induction immunosuppression in heart transplantation. Most of the available studies have been carried out in kidney transplant populations. The recent International Society of Heart and Lung Transplant (ISHLT) guidelines included proposals for the use of rATG but highlighted that these were based largely on expert consensus [5]. To the best of our knowledge, no randomized trial has compared rATG induction versus controls. Three randomized trials had assessed interleukin-2 (IL-2) receptor antagonist induction versus no induction and showed no survival benefit [6–8]. Two randomized trials have compared the use of rATG versus IL-2 receptor antagonist as induction agents in heart transplantations, their results demonstrated a controversial rate of biopsy-proven acute rejection and no long-term survival data were provided [9,10]. Meanwhile, 3 retrospective studies have observed a lower incidence or severity of acute rejection using rATG versus IL-2 receptor antagonist induction [11–13]. These studies had limitations of using fewer than 50 patients and had short-term follow-up periods with no long-term survival data.
Therefore, the long-term survival benefit of rATG induction compared to no induction has not been established due to lack of data and prospective clinical trials. We conducted a retrospective data analysis including 5 year and 10 years follow-up data from 268 consecutive patients who received heart transplants at Tampa General Hospital (TGH). The primary objective of the study was to determine the role of rATG as an induction agent in preventing rejection and improving overall survival. We hypothesized that the induction therapy with rATG will improve overall survival in heart transplant patients.
Material and Methods
Electronic medical records (EMR) were reviewed after an approval from the Tampa General Hospital (TGH) Institutional Review Board was granted for the study. All adult patients 18 years and older, who received a heart transplant at TGH between January 2005 and December 2009 were included.
Immunosuppression
All patients received 500 mg intra-operative then 125 mg every 8 hours for 3 doses of Solu-Medrol, mycophenolate mofetil (MMF), and tacrolimus (TAC). The heart transplant immunosuppressive standard protocol at TGH is a steroid-free maintenance protocol. We defined rATG induction as a patient who received at least 3.0 mg/kg of rATG during the first 72 hours of transplantation. The indication for rATG induction at TGH is the presence of hemodynamic instability associated with acute kidney injury to delay the calcineurin inhibitors initiation and in patients with expected positive cross match.
Outcome measures
The primary outcome of our study was to measure patient’s overall survival. The secondary outcomes were to assess the incidence of rejection, de novo donor specific antibody (DSA) formation after transplantation, rejection grade based on pathology score of first diagnosed rejection reported, tacrolimus levels at the time of first rejection, and the timing of the first rejection episode post-transplantation in rATG induction compared to no rATG induction groups.
Data collection
Data extractions included the date of the heart transplantation, immunosuppressive therapy used at the time of transplantation, date of death, and formation of de novo DSA, date of diagnosed rejection, and tacrolimus level at the time of first biopsy proven rejection.
Statistical analysis
The log-rank test was used to compare the overall survival between rATG induction and no rATG induction groups. Due to the violation of the proportional hazards assumption, the survival differences between groups at 5 years and 10 years were ascertained using restricted mean survival time [14]. Normality of variables was ascertained using the Shapiro-Wilk test. The comparisons were made either using the t-test or Wilcoxon rank sum test for continuous data and chi square or Fisher Exact test for binary data. The results were expressed either as mean ± standard deviation for normally distributed data, median (range) or frequency (percent). P values <0.05 were considered statistically significant. We also assessed the development of de novo DSA after transplantation among the patients (induced with rATG vs. no induction) who were diagnosed with rejection on biopsy findings. All analyses were conducted using STATA 13.1 software.
Results
Survival
One hundred fourteen patients (92%) in the no rATG induction group and 113 patients (79%) in the rATG group were alive after 5 years. At 10 years, 7% were alive for both groups (9 patients in the no rATG, and 10 in the rATG groups). The overall survival was not significantly different between the 2 groups (Figure 1) (P=0.12). However, there was a significant difference in restricted mean survival time (RMST) at 5 years (RMST=4.8 months; 95% CI: 1.0–8.6, P=0.01) and 10 years (RMST=10.4 months; 95% CI: 1.6–19.3, P=0.02) in favor of the no rATG group.
Figure 1.
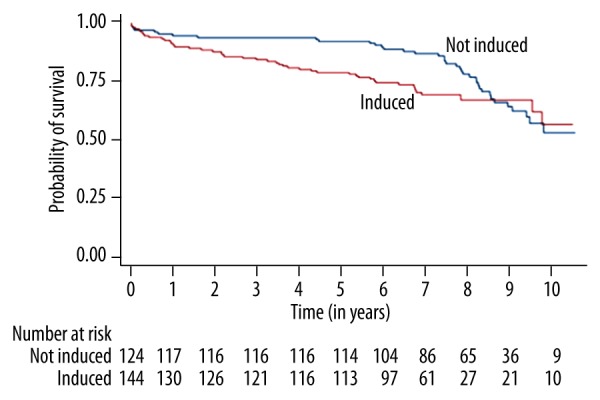
Overall survival in heart transplant patients according to induction vs. no induction group.
De novo DSA
Among the rATG and the no rATG induction groups, 71 patients (49%) and 45 patients (36%) developed DSA, respectively., There was no difference between patients who developed DSA between groups (P=0.10). These findings might suggest that rATG doesn’t protect against DSA associated rejection (Table 1).
Table 1.
Demographic and clinical data.
| Variables | Group 1 (rATG induction n=144) | Group 2 (no rATG induction n=124) | P value |
|---|---|---|---|
| Age | 56.8±10.9 | 54.8±12.4 | 0.15 |
|
| |||
| Sex | 0.78 | ||
| Male | 113 (78.5) | 99 (79.8) | |
| Female | 31 (21.5) | 25 (20.1) | |
|
| |||
| DSA | 0.10 | ||
| Positive | 39 (49.4) | 28 (36.4) | |
| Negative | 40 (50.6) | 49 (63.6) | |
|
| |||
| Number of rejections | 1 (0–5) | 2 (0–6) | 0.90 |
|
| |||
| Time to first rejection (months) | 1.8 (0.2–35.3) | 1.2 (0–22.1) | 0.002 |
|
| |||
| TAC levels | 9.4 (1.2–30) | 9.4 (1.7–24.9) | 0.48 |
|
| |||
| Grading | 0.03 | ||
| Level 1 | 92 (77.3) | 89 (89.0) | |
| Level 2 | 25 (21.0) | 9 (9.0) | |
| Level 3 | 2 (1.7) | 2 (2.0) | |
Data reported as mean ± standard deviation, median (range) or n (%).
Rejection data
When analyzing the data of the first diagnosed rejection, there was no statistical difference in the median number of rejections between non-induced and induced patients (2 vs. 1, respectively, P=0.90). Induction with rATG significantly delayed the incidence of rejection (55 vs. 36 days, respectively, P<0.001). The goal of therapeutic trough level of tacrolimus (TAC) according to the TGH Heart Transplant Program is 8–12 ng/dL. There was no significant difference in TAC levels associated with the first rejection between no rATG versus rATG induction groups (9.4 vs. 9.35, respectively, P=0.483). When analyzing the data of the first diagnosed rejection date; on average, the first rejection episode occurred after 36 days in the no rATG induction compared with an average of 55 days in rATG induction patients. There were statistically significant delays in first diagnosed rejection post-transplantation when rATG was used for induction (P<0.001). The average of TAC levels at the time of first rejection was 9.4 ng/dL and 9.35 ng/dL in the no rATG and the rATG induction groups respectively. The difference was not statistically significant; although these levels indicated adequate adherence to immunosuppression medications. Also, there was no statistical difference in the average number of rejections between the 2 groups. (2 vs. 1, P=0.90).
Discussion
Induction therapy refers to the use of more intense immunosuppression in the initial days after transplantation. The rationale of induction therapy is to provide more intensive immunosuppression at the time when the alloimmune response is most intense [2]. Multiple studies have demonstrated that induction therapy with rATG is an effective means of achieving low rates of acute rejection in most allograft settings in the first and third months post-transplantation [11]. The same conclusion was proven in lung transplantation; where rATG induction reduced the incidence and frequency of early acute rejection compared with no induction therapy [15]. Induction therapy permits the delayed initiation of calcineurin inhibitors (CNIs) for maintenance immunosuppression in patients with significant renal failure [16]. There is good evidence that rATG induction with reduced exposure to CNI and steroids will provide similar efficacy to a conventional CNI regimen [16,17]. Another known benefit is that the induction with rATG lowers the incidence of angiographic cardiac allograft vasculopathy [18,19].
Although in our study there was no difference in the average number of rejections between the 2 groups, there was a statistically significant delay in first diagnosed rejection from 36 days to 55 days post-transplantation when rATG was used for induction (P<0.001). Another interesting finding of our study was that there was no difference in TAC levels during the first rejection between the 2 groups.
Awareness of the prognostic importance of DSAs is influencing initial immunosuppressive protocols according to patients’ pre-transplantation DSA status. Recipients with pre-transplantation DSA are far more likely to experience antibody-mediated or cellular rejection and graft failure despite desensitization measures, which is hard to apply to heart transplantation as the pool of organs is smaller, heart grafts are less well-matched than other organs, transplantation is more urgent, and graft storage times are shorter, and it is often not possible to await the results of cross-match testing [16,20–23].
In our study, there was no difference in de novo DSA formation between the groups. These findings suggest that rATG doesn’t protect against de novo DSA associated rejection. There is little evidence looking at the effects of rATG on de novo DSA production and risk of antibody-mediated rejection (AMR) in heart transplantation. Prospective studies are required to assess DSA recurrence after desensitization, rates of AMR in pre-sensitized or otherwise high-risk individuals, and the development of de novo DSA. More generally, the challenge remains to identify accurate criteria to define “high risk” for de novo DSA or for AMR other than pre-transplantation DSA [16].
The impact of induction immunosuppression on long-term survival in heart transplant recipients is unclear; however, the trials performed to date have either shown that specialized induction agents have modest benefits over regimens with non-specialized induction or have been associated with increased morbidity [24]. Higgins et al. evaluated a multi-institutional database and found that the survival benefit for induction agents was seen when the individual risk of 1-year mortality was >5% [25]. Additionally, these investigators reported a low risk cohort (risk of death <2%) that did not have a survival benefit. Patients with ventricular assisted device support, African American ethnicity, and increased HLA mismatching were at higher risk of death and benefited from induction therapy. In our study, we confirmed their findings that induction with rATG adds no survival benefit in heart transplant recipients. However, patients who did not receive rATG induction therapy had higher life expectancy at 5 years and 10 years post-transplantation, this has not been shown previously.
The present study has several limitations. It was a retrospective cohort study and the number of patients included in both groups was limited. Patients were not randomized to receive induction therapy; therefore, differences in the baseline clinical characteristics of our population may partially explain our results. Significant limitation appears when comorbidities data peri-transplantation were not collected since the intention of the study was to address the long-term survival related to induction. We believe that a randomized trial to address the peri-transplantation comorbidities in relation to long-term survival is needed. A trial should also address all potential infectious and malignant complications associated with heart transplantation.
Conclusions
The proper use of induction immunosuppression is still being determined. Modern immunosuppressive regimens should be individualized. Our study suggests that induction with rATG adds no survival benefit in heart transplant recipients. Furthermore, patients who did not receive rATG induction therapy had longer life expectancy at 5 years and 10 years post-transplantation. Although, there was a significant delay in the first rejection episode in favor of rATG induction group, DSAs were not significantly different between the 2 groups. This study indicates a need for separate analysis of peri-transplantation co-morbidities, such as the incidence of acute kidney injury, which could affect long-term survival.
Acknowledgement
The authors thank Dr. Christiano Caldeira and Dr. Athar Naif for their contribution in providing valuable information towards the completion of this manuscript
Abbreviations
- MMF
mycophenolate mofetil
- TAC
tacrolimus
- CNI
calcineurin inhibitor
- rATG
rabbit anti-thymocyte globulin
- DSA
donor specific antibodies
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Kirk AD. Induction immunosuppression. Transplantation. 2006;82(5):593–602. doi: 10.1097/01.tp.0000234905.56926.7f. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Haddad F. The changing face of heart transplantation. J Am Coll Cardiol. 2008;52(8):587–98. doi: 10.1016/j.jacc.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 3.Lund LH, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Official Adult Heart Transplant Report – 2013; focus theme: Age. J Heart Lung Transplant. 2013;32(10):951–64. doi: 10.1016/j.healun.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Dipchand AI, Kirk R, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Heart Transplantation Report – 2013; focus theme: Age. J Heart Lung Transplant. 2013;32(10):979–88. doi: 10.1016/j.healun.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Cantrelle C, Legeai C, Latouche A, et al. Access to heart transplantation: a proper analysis of the competing risks of death and transplantation is required to optimize graft allocation. Transplant Direct. 2017;3(8):e198. doi: 10.1097/TXD.0000000000000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehra MR, Zucker MJ, Wagoner L, et al. A multicenter, prospective, randomized, double-blind trial of basiliximab in heart transplantation. J Heart Lung Transplant. 2005;24(9):1297–304. doi: 10.1016/j.healun.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Beniaminovitz A, Itescu S, Lietz K, et al. Prevention of rejection in cardiac transplantation by blockade of the interleukin-2 receptor with a monoclonal antibody. N Engl J Med. 2000;342(9):613–19. doi: 10.1056/NEJM200003023420902. [DOI] [PubMed] [Google Scholar]
- 8.Hershberger RE, Starling RC, Eisen HJ, et al. Daclizumab to prevent rejection after cardiac transplantation. N Engl J Med. 2005;352(26):2705–13. doi: 10.1056/NEJMoa032953. [DOI] [PubMed] [Google Scholar]
- 9.Carrier M, Leblanc MH, Perrault LP, et al. Basiliximab and rabbit anti-thymocyte globulin for prophylaxis of acute rejection after heart transplantation: A non-inferiority trial. J Heart Lung Transplant. 2007;26(3):258–63. doi: 10.1016/j.healun.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Haddad H, Isaac D, Legare JF, et al. Canadian Cardiovascular Society Consensus Conference update on cardiac transplantation 2008: Executive summary. Can J Cardiol. 2009;25(4):197–205. doi: 10.1016/s0828-282x(09)70061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado DH, Miriuka SG, Cusimano RJ, et al. Use of basiliximab and cyclosporine in heart transplant patients with pre-operative renal dysfunction. J Heart Lung Transplant. 2005;24(2):166–69. doi: 10.1016/j.healun.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 12.Ansari D, Hoglund P, Andersson B, Nilsson J. Comparison of basiliximab and anti-thymocyte globulin as induction therapy in pediatric heart transplantation: A survival analysis. J Am Heart Assoc. 2015;5(1) doi: 10.1161/JAHA.115.002790. pii: e002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mullen JC, Kuurstra EJ, Oreopoulos A, et al. A randomized controlled trial of daclizumab versus anti-thymocyte globulin induction for heart transplantation. Transplant Res. 2014;3:14. doi: 10.1186/2047-1440-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–97. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- 15.Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis. 2014;6(8):1039–53. doi: 10.3978/j.issn.2072-1439.2014.04.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuckermann A, Schulz U, Deuse T, et al. Thymoglobulin induction in heart transplantation: Patient selection and implications for maintenance immunosuppression. Transpl Int. 2015;28(3):259–69. doi: 10.1111/tri.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreassen AK, Andersson B, Gustafsson F, et al. Everolimus initiation and early calcineurin inhibitor withdrawal in heart transplant recipients: A randomized trial. Am J Transplant. 2014;14(8):1828–38. doi: 10.1111/ajt.12809. [DOI] [PubMed] [Google Scholar]
- 18.Carrier M, White M, Perrault LP, et al. A 10-year experience with intravenous thymoglobuline in induction of immunosuppression following heart transplantation. J Heart Lung Transplant. 1999;18(12):1218–23. doi: 10.1016/s1053-2498(99)00100-x. [DOI] [PubMed] [Google Scholar]
- 19.Wang R, Moura LA, Lopes SV, et al. Reduced progression of cardiac allograft vasculopathy with routine use of induction therapy with basiliximab. Arq Bras Cardiol. 2015;105(2):176–83. doi: 10.5935/abc.20150063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinsmoen NL, Lai CH, Mirocha J, et al. Increased negative impact of donor HLA-specific together with non-HLA-specific antibodies on graft outcome. Transplantation. 2014;97(5):595–601. doi: 10.1097/01.TP.0000436927.08026.a8. [DOI] [PubMed] [Google Scholar]
- 21.Ho EK, Vlad G, Vasilescu ER, et al. Pre- and posttransplantation allosensitization in heart allograft recipients: Major impact of de novo alloantibody production on allograft survival. Hum Immunol. 2011;72(1):5–10. doi: 10.1016/j.humimm.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Eckman PM. Immunosuppression in the sensitized heart transplant recipient. Curr Opini Organ Transplant. 2010;15(5):650–56. doi: 10.1097/MOT.0b013e32833de9b2. [DOI] [PubMed] [Google Scholar]
- 23.Fine NM, Daly RC, Shankar N, et al. The role of donor-specific antibodies in acute cardiac allograft dysfunction in the absence of cellular rejection. Transplantation. 2014;98(2):229–38. doi: 10.1097/TP.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherikh WS, Kauffman HM, McBride MA, et al. Association of the type of induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation. 2003;76(9):1289–93. doi: 10.1097/01.TP.0000100826.58738.2B. [DOI] [PubMed] [Google Scholar]
- 25.Higgins R, Kirklin JK, Brown RN, et al. To induce or not to induce: do patients at greatest risk for fatal rejection benefit from cytolytic induction therapy? J Heart Lung Transplant. 2005;24(4):392–400. doi: 10.1016/j.healun.2004.01.002. [DOI] [PubMed] [Google Scholar]


