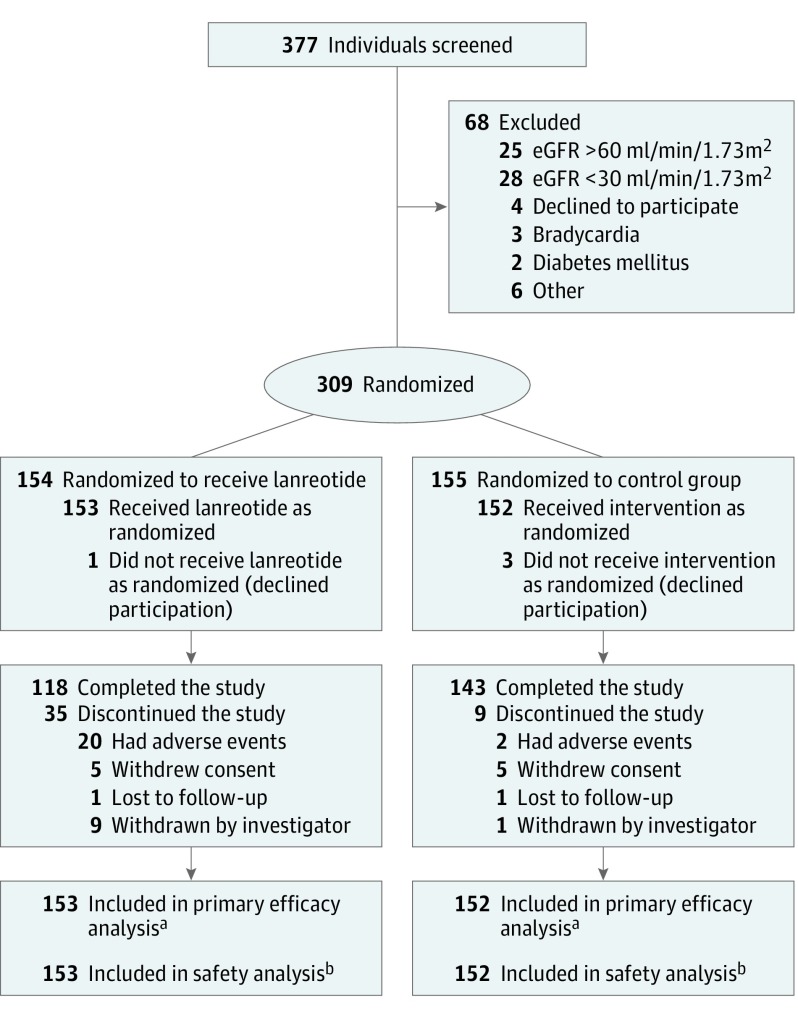
Figure 1. Patient Enrollment and Flow Through the Study of Lanreotide for Polycystic Kidney Disease.
Enrollment occurred from July 2012 to February 2014, and follow-up of the 2.5-year trial was completed in August 2017. Supplement 3 contains additional information regarding specific reasons for exclusion and withdrawal.
aIf patients did not complete the study, eGFR data for the primary efficacy analysis (slope of eGFR decline on treatment) was based on all available data points as long as medication was given.
bFor the safety analysis, all information was used until patients withdrew consent or were lost to follow-up.