Abstract
Background
Patients undergoing re-transplantation often receive high doses of immunosuppression, which may lead to an immunocompromised status of the recipient. This study investigates the outcomes after intestine/multivisceral re-transplantation.
Material/Methods
Clinical outcomes of 23 patients undergoing 24 re-transplantations at a single intestine transplant center were reviewed. Bone marrow suppression was used as a surrogate marker of immunocompromised status, and was defined as platelet count <50 k/mm3 and absolute lymphocyte count <200/mm3.
Results
All re-transplants except one were liver inclusive. Fifteen of 23 patients died at a median time of 12 months (range 0.2–75) after re-transplantation. Of the 15 deaths, nine (60%) resulted from complications associated with a compromised host immune status: graft versus host disease (GVHD) affecting bone marrow (three cases), persistent viral infection (three cases), post-transplant lymphoproliferative disorder (PTLD (one case), metastatic cancer (one case), multi-drug resistant polymicrobial sepsis (one case). Four deaths (27%) resulted from severe rejection. Non-survivors were more likely to have received alemtuzumab, and had higher incidence of bone marrow suppression. In addition to immunocompromised status and rejection, the use of alemtuzumab was associated with mortality after intestinal/multivisceral re-transplantation.
Conclusions
High mortality was associated with intestine/multivisceral re-transplantation. To improve clinical outcomes of intestine and multivisceral transplantation, it is important to allow reconstitution of host immunity. Longer interval between the two transplantations, and strategies such as allograft specific immunosuppression, may spare the host from the devastating effects of potent immunosuppression currently used.
MeSH Keywords: Graft Rejection, Graft vs Host Disease, Immunocompromised Host, Intestine, Small, Transplantation
Background
Acute and chronic rejection continue to be major impediments to success after intestine and multivisceral transplantation. Intestine or multivisceral re-transplant is now considered as a therapeutic option in many patients with primary graft loss. A review of United States intestine transplant volume from 2016 indicates that 16% were re-transplants [optn.transplant.hrsa.gov]. Potent induction and maintenance immunosuppression is used in primary intestine and multivisceral transplantation. When severe rejection occurs, strong immunosuppressive agents, such as alemtuzumab and rabbit anti-thymocyte globulin (rATG), are administered in hopes of reversing the rejection. When treatment of rejection fails, and patients receive a re-transplant, they often receive another round of induction immunosuppression. This immunosuppressive therapy exposes the transplant recipient to a large cumulative dosage of biologic immunosuppression, which often leads to a severely immunocompromised state. Bone marrow suppression, thrombocytopenia, and leukopenia are observed with repeated doses of anti-lymphocyte therapy [1]. Infections, including opportunistic infections and viral infections, and malignancies are common in immunocompromised patients and are often associated with morbidity and mortality. Thus, intestine re-transplant recipients have a significant risk from infections and malignancies.
Previous reports have suggested a range of outcomes after intestinal re-transplantation [2–6]. Some studies, including a paper based on OPTN data [2], have reported worse clinical outcomes for re-transplant cases when compared to primary transplant cases. Other studies have reported improved outcomes, mainly attributed to improvements in immunosuppression protocols, proper timing, and improved infectious disease monitoring [3]. As there has been no significant improvement in long-term graft survival following intestine and multivisceral transplantation, re-transplantation will be an important component of care for these patients. With advanced surgical techniques, overall short-term outcomes have improved. Thus, to improve long-term outcomes, it is important to understand the immunologic aspects of intestine and multivisceral re-transplantation, in order to avoid over-immunosuppression.
Material and Methods
Patients
The records of all patients undergoing intestine and multivisceral re-transplantation between 2005 and 2016 were reviewed. Retrospective analysis of patient data from the transplant center database was reviewed and approved by the Institutional Review Board at the Indiana University School of Medicine. Records were analyzed for transplant indications, operative techniques, immunosuppression, complications, and outcomes. Immune system-related complications in the form of rejection, graft versus host disease (GVHD), bone marrow suppression, and lymphopenia were analyzed.
Surgical procedure
The surgical re-transplant procedure in each case was very similar to the primary transplant, as previously described [7], except that the re-transplantation included explant of the allograft in cases where allograft enterectomy was not previously performed [8]. In cases of prior multivisceral transplant, the aortic conduit from the first transplant was used again for arterial inflow, with outflow through the hepatic veins. All re-transplants, except one, were liver inclusive multivisceral transplants. Terminal ileostomy with ileocolic anastomosis was formed when possible. The ileostomy was taken down between six and 12 months after transplantation. In the most recent nine re-transplants (since May 2013), donor colon was included in the allograft, and when possible, donor to recipient colo-colic anastomosis was performed at the time of transplantation, without use of a temporary protective ostomy.
Immunosuppression
Patients received induction immunosuppression in the form of rATG (three or five doses of 2 mg/kg for adults, and four doses of 2 mg/kg for pediatric recipients) and rituximab at a single dose of 150 mg/m2 body surface area (BSA), with a rapid methylprednisolone taper [9]. The rATG dosage was reduced from five to three doses of 2 mg/kg in all recipients in January 2013 (11 re-transplant recipients). Maintenance immunosuppression was tacrolimus with steroids. Target trough tacrolimus levels were between 8 ng/dL and 12 ng/dL, which were maintained for the first three months and then decreased to 6 ng/dL to 10 ng/dL, thereafter. An anti-IL2 receptor antibody (basiliximab 40 mg or daclizumab 1 mg/kg) was added to the maintenance immunosuppression protocol in patients not receiving a liver graft, or in the presence of rejection.
Treatment of rejection
Patients with mild rejection episodes received pulse methylprednisolone with a taper. Patients with moderate and severe rejection received one or two doses of rATG (2 mg/kg), along with a methylprednisolone taper. Some patients with severe rejection episodes, which did not improve with rATG and steroid taper, also received one or two doses of alemtuzumab. Infliximab was used in patients with exfoliative rejection early on after transplantation. Patients with rejection in the presence of donor specific antibody (DSA) with median fluorescence intensity (MFI) greater than 5,000 received plasmapheresis and four cycles of bortezomib (each cycle consisting of four 1.3 mg/m2 body surface area (BSA) doses on days 1, 4, 8, and 11).
Crossmatch and analysis of anti-HLA antibodies
Flow cytometry crossmatch (FCCM) was used in this study. A positive FCCM was defined as a shift in the mean channel of fluorescence of more than 50 channels to the right for the T-cell peak and more than 150 channels for the B-cell peak in the test sera, compared to the negative control. A positive result due to non-donor specific antibody (NDSA) was considered as a negative crossmatch. Transplantations were performed regardless of crossmatch status. Donor-specific anti-human leukocyte antigen (HLA) antibodies were tested as per our clinical practice at the time of transplantation and at 1, 3, 6, 9, 12, 18, 24, and 36 months after transplantation.
Study endpoints
The primary endpoint of this study was patient survival. Secondary endpoints included bone marrow suppression, which is a marker of severely immunocompromised status. Bone marrow suppression was defined as platelet count <50 k/mm3 and absolute lymphocyte count <200/mm3. Standard statistical testing was conducted using commercially available software (IBM SPSS Statistics, Version 24.0. Armonk, NY, USA). Categorical variables were compared using chi square or Fisher exact test, as appropriate. Continuous variables were compared using Mann-Whitney U Test or student’s t-test. Kaplan-Meier survival curves were constructed with Log-rank testing for group differences. A p-value <0.05 was considered statistically significant.
Results
Of 246 intestine and multivisceral transplantations during the study period, there were 24 re-transplantations in 23 patients (10%). One patient underwent two re-transplantations. Seventy percent of the recipients were adults. The median age at first transplantation was 26 years [range, 0.3–56 years], and 27 years [range, 1–58 years] at the time of re-transplantation. Indications for re-transplantation were early exfoliative rejection in six cases (within four weeks from transplantation), late exfoliative rejection in nine cases, chronic rejection in three cases, allograft pancreatitis in two cases, severe intestine dysmotility in two cases, aneurysmal dissection of aortic conduit in one case, and persistent duodenal fistula in one case. The median time of first graft survival was 15 months (range, 12 days to 8.4 years). Overall, 15 of 23 patients died at a median time of 12 months after re-transplantation (range, 0.2 to 75 months).
Patient demographics, stratified by survival outcomes, are listed in Table 1. Among pediatric recipients of re-transplants, survival was 57%, whereas survival was only 25% among adult recipients of re-transplants. The type of first transplant did not affect patient survival. Rejection as the cause of primary graft loss was numerically higher in liver-excluding primary transplants (82% versus 50%, p=0.12). Liver inclusion in the primary transplant did not affect overall patient survival. Crossmatch was positive in seven transplants (30%), whereas in five transplants, de novo DSA was identified (22%). Crossmatch status and occurrence of de novo DSA were comparable between survivors and non-survivors.
Table 1.
Patient demographic, pre-transplant and post-transplant characteristics stratified by long-term survival.
| Patients with long-term survival n=8 | Patients without long-term survival n=15 | p | |
|---|---|---|---|
| Age | 16 [4–40] | 30 [1–58] | |
| Adult: Pediatric | 4: 4 | 12: 3 | 0.14 |
| Gender (M: F) | 3: 5 | 6: 9 | 0.9 |
| Race | |||
| Caucasian/African American/other | 6: 0: 2 | 12: 1: 2 | 0.62 |
| Type of primary transplant | |||
| IIT/MMVT/MVT | 5/2/1 | 6/4/5 | 0.49 |
| Cause of primary graft loss | |||
| Rejection | 7 (88%) | 10 (67%) | 0.28 |
| Non-rejection | 1 (12%) | 5 (33%) | |
| Enterectomy | |||
| At re-transplant | 6 (75%) | 12 (80%) | 0.78 |
| Prior to re-transplant | 2 (25%) | 3 (20%) | |
| Positive crossmatch | 2 (25%) | 5 (33%) | 0.45 |
| CMV risk | 0.4 | ||
| Low (−/−) | 2 (25%) | 6 (40%) | |
| Low intermediate (−/+) | 4 (50%) | 3 (20%) | |
| High intermediate (+/+) | 1 (12.5%) | 5 (33%) | |
| High (+/−) | 1 (12.5%) | 1 (7%) | |
| Pre re-transplant characteristics | |||
| Bone marrow suppression | 0 | 1 (7%) | 0.56 |
| Absolute lymphocyte count | 1.1 [0.2–8] | 0.9 [0–6.7] | 0.39 |
| Thrombocyte count | 209 [17–281] | 209 [15–626] | 1 |
| Absolute CD4+ cells | 187 [37–441] | 171 [13–652] | 0.52 |
| Absolute CD8+ cells | 425 [157–1158] | 311 [10–940] | 0.61 |
| Absolute CD19+ cells | 100 [19–784] | 88 [0–1781] | 0.7 |
| Absolute CD16+CD56+ cells | 107 [80–771] | 91 [10–646] | 0.44 |
| Viral infection | 3 (37%) | 9 (60%) | 0.6 |
| Interval between transplants (median months) | 40 [1–82] | 13 [1–101] | 0.15 |
| Post re-transplant characteristics | |||
| Rejection | 2 (25%) | 6 (40%) | 0.47 |
| Bone marrow suppression | 0 | 8 (53%) | 0.01 |
| GVHD | 0 | 6 (40%) | 0.06 |
| Viral infection | 4 (50%) | 9 (60%) | 0.65 |
| PTLD | 3 (38%) | 2 (13%) | 0.18 |
| Median absolute lymphocyte count* | 3.2 [2.2–7.5] | 0.2 [0–1.4] | 0.0001 |
| Median platelet count* | 508 [332–936] | 42 [0–495] | 0.0001 |
| De novo DSA | 2 (25%) | 3 (20%) | 0.78 |
| Other cancer | 0 | 1 (7%) | 0.46 |
| Death caused by immunocompromised status | 0 | 9 (60%) | 0.005 |
| Death caused by rejection | 0 | 5 (33%) | 0.07 |
Cell counts at 2–4 weeks prior to death in non-survivors were compared with those at 12 months post-transplant period in survivors.
IIT – isolated intestine transplant; MMVT – modified multivisceral transplant; MVT – multivisceral transplant.
Median time interval between the first and second transplantations was higher in the survivors, however, this did not reach statistical significance (40 months (range 1–82 months) versus 13 months (range, 1–101 months); p=0.15). Allograft enterectomy was performed prior to re-transplantation in six patients at a median period of 85 days prior to re-transplantation [range 30–252 days]. This finding again did not have any impact on patient survival. All patients in the cohort received a similar induction protocol, and total immunosuppression received prior to re-transplantation was comparable between survivors and non-survivors. Median dosage of rATG and rituximab was comparable between the two groups (Table 2). Allograft enterectomy was performed prior to re-transplantation in only 6 patients.
Table 2.
Immunosuppressive therapy utilized in patients stratified by long-term survival.
| Patients with long-term survival (n=8) | Patients without long-term survival (n=15) | P | |
|---|---|---|---|
| Cumulative antibody therapy | |||
| rATG (mg/kg), mean ±SD | 16.7±5.1 | 19±6.5 | 0.41 |
| Rituximab dosage (mg/m2), mean ±SD | 439.8±97.3 | 409.5±115.4 | 0.67 |
| Anti-IL 2ab (doses), mean ±SD* | 10±5.1 | 9.6±3.6 | 0.62 |
| Alemtuzumab (mg), mean ±SD | 0 | 14.4±5.6 | 0.001 |
| Pre re-transplant antibody therapy | |||
| rATG dosage (mg/kg), mean ±SD | 8.9±2.8 | 9.4±2.7 | 0.71 |
| Rituximab dosage (mg/m2), mean ±SD | 291.6±85 | 148.4±2.3 | 0.01 |
| Anti-IL 2ab (doses), mean ±SD* | 3±1.6 | 6.1±2.2 | 0.56 |
| Alemtuzumab (mg), mean ±SD | 0 | 14.4±5.6 | 0.001 |
| Post re-transplant antibody therapy | |||
| rATG dosage (mg/kg), mean ±SD | 7.8±3.3 | 9.6±4.6 | 0.37 |
| Rituximab dosage (mg/m2), mean ±SD | 148.2±32.7 | 261.1±113.9 | 0.56 |
| Anti-IL 2ab (doses), mean ±SD* | 7±5 | 3.5±2.2 | 0.28 |
| Alemtuzumab (mg), mean ±SD | 0 | 0 | na |
Anti-IL 2 dosage: Daclizumab 2 mg/kg; Basiliximab 40 mg for adults, 20 mg for >25 kg, 10 mg/kg for <25 kg.
rATG – rabbit anti-thymocyte globulin.
Immunosuppression
Median pre- re-transplant doses of rATG and rituximab were comparable between the two groups. Cumulatively, pre and post re-transplant exposure to antibody therapy is summarized in Table 2. Most strikingly, the use of alemtuzumab prior to re-transplantation was associated with mortality; all patients receiving this drug as part of anti-rejection therapy died, whereas none of the survivors received it (p=0.02). Alemtuzumab was not used after re-transplantation in the entire cohort. Patients with long-term survival, however, received significantly more rituximab prior to re-transplantation (p=0.03). The three pediatric cases in this group of long-term survivors received increased doses of rituximab: one for DSA, one for hemolytic anemia, and one for PTLD. More patients in the long-term survival group received anti-IL2 receptor antibody maintenance immunosuppression and infliximab treatment for rejection. However, these findings were not statistically significant (83% versus 40%; p=0.15, and 67% versus 27%; p=0.15, respectively). One patient in the non-survivor group received two cycles of bortezomib, one after each transplantation. As allograft enterectomy was performed prior to re-transplantation in only six patients, the immunosuppression free period did not impact patient survival. However, the median time period between the two transplantations was numerically higher in survivors (40 months (range, 1–82 months) versus 13 months (range, 1–101 months); p=0.15).
Immunologic outcomes
Recurrent severe rejection was common even after re-transplantation, occurring in eight patients (35%). The incidence of rejection of the re-transplant graft was significantly higher in patients who had rejection of their first transplant leading to graft loss (8 of 17 (47%) versus 0%, p=0.04). Mortality was slightly higher in patients developing rejection after re-transplantation (75% versus 60%; p=0.50). T-cell and B-cell repertoire was available for analysis only prior to re-transplantation. These were comparable between the survivors and non-survivors (Table 1).
Severely immunocompromised state was common after re-transplantation. Bone marrow suppression was observed in 35% of re-transplant patients compared to 4% of first transplant patients (p=0.01). Bone marrow suppression was associated with mortality (Figure 1). Median post re-transplantation absolute lymphocyte counts one month prior to death in non-survivors were significantly lower than those seen at 12 months (comparable timeline) post re-transplantation in survivors (0.2, (range, 0–1.4) versus 3.2 (range, 2.2–7.5); p<0.001). Similarly, median post re-transplant platelet counts one month prior to death in non-survivors were significantly lower than those at 12 months post re-transplant in survivors (42 (range, 0–495) versus 508 (range, 332–936); p<0.001). In two patients, severe thrombocytopenia was associated with spontaneous intracerebral hemorrhage leading to death. PTLD was also common after re-transplantation (22% versus 9%; p=0.01), however diagnosis of PTLD was not associated with mortality in this study. Viral infection was present in 57% of patients after re-transplantation, whereas tissue invasive fungal infection was present in 13% of patients. GVHD was also common after re-transplantation, occurring in six patients (26%). In two patients, the GVHD involved bone marrow, causing severe bone marrow suppression.
Figure 1.
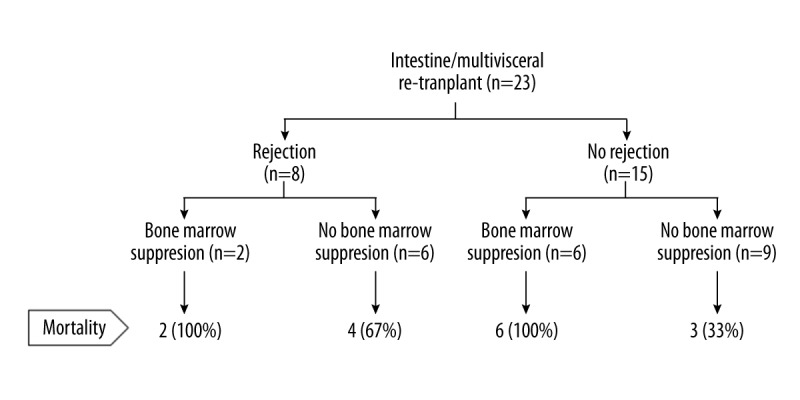
Schematic representation of the clinical outcomes with reference to immunologic complications.
Death analysis
Fifteen of 23 patients died at a median period of 12 months after re-transplantation (range 0.2–75 months). Causes of death were severe rejection (six cases), GVHD affecting bone marrow (three cases), persistent viral infection (three cases), PTLD (one case), metastatic cancer (one case), and multi-drug resistant polymicrobial sepsis (one case). The use of alemtuzumab, bone marrow suppression (Figure 1), GVHD, and death due to immunocompromised state were higher in non-survivors.
Discussion
In this study, we found poor outcomes after intestine and multivisceral re-transplantation, which provides an opportunity to understand the nature of complications associated with this procedure. A review of these complications is of use to both established and emerging programs. The Intestine Transplant Registry recently reported an 8% re-transplantation rate, and second or third graft survival rates of 56% at one year and 35% at five years [10]. Considering high graft loss rates after intestine and multivisceral transplantation, re-transplantation is and will be used in this organ setting more than other solid organ transplant settings. For this reason, it is important to understand the pathophysiology, particularly from patient selection and immunologic perspective.
Mazariegos et al. have reported improved outcomes in pediatric intestine and multivisceral re-transplantation and have attributed the improvement to improvement in patient selection, immunosuppression, viral monitoring, and patient management [3]. Patient’s clinical condition prior to intestine and multivisceral transplantation is important to successful outcomes, and this applies even more so in the setting of re-transplantation [10]. In a pediatric series, Mazariegos et al. suggested that the most favorable outcomes were associated with a clinical status that allowed the patient to be at home before re-transplantation [3]. In our series, only nine patients (41%) were at home at the time of re-transplantation, and of 13 patients that were in the hospital at the time of re-transplantation, 11 patients (85%) died. It is likely that poor outcomes in our series were related to poor patient selection, and that some patients were beyond a salvageable stage even with re-transplantation.
It has been shown that allograft enterectomy can be lifesaving and improves recovery from comorbidities [8]. In our series, interval allograft enterectomy was performed in six re-transplantations (28%) at a median interval of 85 days. Although this immunosuppression free interval might have allowed adequate recovery time from life-threatening complications, the time was perhaps inadequate for reconstitution of the recipient immune system. Consequently, complications associated with immunocompromised status were common, and were fatal in nine patients.
In this study, we demonstrate that immunologic consequences play a major role in the mortality associated with intestine and multivisceral re-transplantation. The majority of deaths in this cohort resulted from complications of immunosuppression, from rejection on one end of the immunologic spectrum to complications of over-immunosuppression on the other end. Interestingly, rejection occurred in eight patients despite receiving two rounds of induction immunosuppression, and potent anti-rejection therapy. Rejection was the cause of primary graft loss in these eight patients. And including liver in the allograft was not protective against rejection in these patients. At the other end of the spectrum, complications associated with immunocompromised status were also common. GVHD was observed in 12 patients (57%), viral infections in six patients (29%) and PTLD in five patients (24%). Except for PTLD, all other complications were associated with mortality.
We used absolute lymphocyte count and platelet count to define bone marrow suppression as a surrogate marker of immune status. Persistent lymphopenia has been shown to be associated with mortality after intestine and multivisceral transplantation, particularly infection associated mortality [11]. In this study, we defined bone marrow suppression as absolute lymphocyte count < 200/mm3. This threshold was even lower than 500/mm3 used in previous studies on lymphopenia [11]. Unfortunately, we did not have T-cell and B-cell repertoire after re-transplantation to characterize the immune reconstitution. Theoretically, it makes sense to perform enterectomy and allow the patient to reconstitute the immune system in the absence of immunosuppression. However, it is not always possible to perform enterectomy, particularly in patients with modified or full multivisceral transplants. In the report from University of Pittsburgh with better outcomes after re-transplantation, the majority of re-transplant recipients were allowed prolonged immunosuppression free intervals [3].
To prevent over-immunosuppression in re-transplant candidates, it is paramount to allow reconstitution of the immune system prior to re-transplantation. When this is not possible, immunosuppression regimens may need to be customized to their individual condition. It may be prudent to avoid potent induction immunosuppression in such recipients. Wu and Cruz, in their series of 23 re-transplant cases, reported that 43% of recipients did not receive any induction therapy [5]. However, in the absence of induction therapy, the donor passenger lymphocytes can potentially overwhelm the weaker host and cause GVHD. To prevent this possibility, a combination of donor pre-treatment with potent antibody therapy prior to re-transplantation may be considered. Another potential solution may be to use intestine specific immunosuppression such as vedolizumab, a monoclonal antibody selectively blocking intestinal lymphocyte migration while treating rejection episodes and after re-transplantation to preserve host immunity [12]. In our study, use of alemtuzumab prior to re-transplantation was associated with mortality. This is indicative of the overall potency of the immunosuppression received. Alemtuzumab has also been shown to disrupt intestinal barrier function in animal studies, which may cause sepsis [13].
Ultimately, the decision of performing re-transplantation and the timing are crucial in this setting. Performing liver-including re-transplantation for immunologic protection was not successful in our case series, which raises an important question around this strategy. It may be prudent to perform isolated intestinal re-transplantations and have the option of allograft enterectomy in life-threatening situations. It must be noted that performing a second re-transplantation after a failed liver-including re-transplantation is usually not feasible. It is clear from this study that identifying suitable candidates for re-transplantation is extremely important. Utilizing thorough immunologic assessment of host immunity is important prior to re-transplantation and perhaps there is a need to tailor post re-transplantation immunosuppression based on this assessment instead of using program based protocols.
Conclusions
To improve clinical outcomes of intestine and multivisceral transplantation, it is important to allow reconstitution of host immunity. This may be achieved by allowing prolonged immunosuppression free periods prior to re-transplantation or using individualized immunosuppression strategies guided by patients’ immunocompetency. The concept of liver inclusion for immunologic benefits may need to be revisited. And finally, re-transplantation may not be feasible in every recipient with failed primary intestinal/multivisceral transplant.
Footnotes
Source of support: Departmental sources
References
- 1.Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48(6):772–86. doi: 10.1086/597089. [DOI] [PubMed] [Google Scholar]
- 2.Desai CS1, Khan KM, Gruessner AC, et al. Intestinal retransplantation: Analysis of Organ Procurement and Transplantation Network database. Transplantation. 2012;93(1):120–25. doi: 10.1097/TP.0b013e31823aa54d. [DOI] [PubMed] [Google Scholar]
- 3.Mazariegos GV, Soltys K, Bond G, et al. Pediatric intestinal retransplantation: techniques, management, and outcomes. Transplantation. 2008;86(12):1777–82. doi: 10.1097/TP.0b013e3181910f51. [DOI] [PubMed] [Google Scholar]
- 4.Trevizol AP, David AI, Yamashita ET, et al. Intestinal and multivisceral retransplantation results: Literature review. Transplant Proc. 2013;45(3):1133–36. doi: 10.1016/j.transproceed.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Cruz RJ. Liver inclusion improves outcomes of intestinal retransplantation in adults. [Corrected] Transplantation. 2015;99(6):1265–72. doi: 10.1097/TP.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 6.Grant D, Abu-Elmagd K, Reyes J, et al. 2003 report of the intestine transplant registry: A new era has dawned. Ann Surg. 2005;241(4):607–13. doi: 10.1097/01.sla.0000157265.85388.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vianna R, Kubal C, Mangus R, et al. Intestinal and multivisceral transplantation at Indiana University 6 years’ experience with 100 cases. Clin Transpl. 2009:219–28. [PubMed] [Google Scholar]
- 8.Nagai S, Mangus RS, Anderson E, et al. Intestinal graft failure: Should we perform the allograft enterectomy before or with retransplantation? Transplantation. 2017;101(2):411–20. doi: 10.1097/TP.0000000000001102. [DOI] [PubMed] [Google Scholar]
- 9.Vianna RM, Mangus RS, Fridell JA, et al. Induction immunosuppression with thymoglobulin and rituximab in intestinal and multivisceral transplantation. Transplantation. 2008;85(9):1290–93. doi: 10.1097/TP.0b013e31816dd450. [DOI] [PubMed] [Google Scholar]
- 10.Grant D, Abu-Elmagd K, Mazariegos G, et al. Intestinal transplant registry report: Global activity and trends. Am J Transplant. 2015;15(1):210–19. doi: 10.1111/ajt.12979. [DOI] [PubMed] [Google Scholar]
- 11.Nagai S, Mangus RS, Anderson E, et al. Post-transplant persistent lymphopenia is a strong predictor of late survival in isolated intestine and multivisceral transplantation. Transpl Int. 2015;28(10):1195–204. doi: 10.1111/tri.12620. [DOI] [PubMed] [Google Scholar]
- 12.Norsa L, Francisca J, Busch A, et al. Vedolizumab after intestinal transplantation. Transplantation. 2017;101(6S2):S116. [Google Scholar]
- 13.Qu LL, Lyu YQ, Jiang HT, et al. Effect of alemtuzumab on intestinal intraepithelial lymphocytes and intestinal barrier function in cynomolgus model. Chin Med J (Engl) 2015;128(5):680–86. doi: 10.4103/0366-6999.151675. [DOI] [PMC free article] [PubMed] [Google Scholar]


