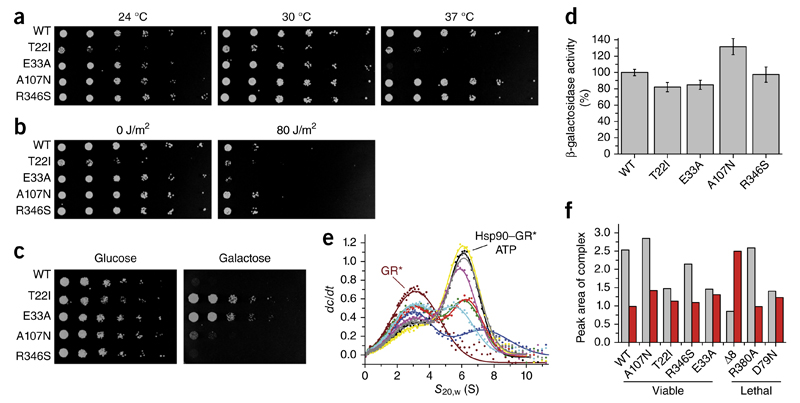
Figure 6.
Influence of Hsp90 variants in vivo. (a) Temperature sensitivity of yeast cells expressing Hsp90 WT and variants as the sole Hsp90 source at 24 °C, 30 °C or 37 °C. The assay was carried out at least three times, and data represent technical replicates from independent single yeast colonies. (b) Influence of the Hsp90 variants on DNA-repair activity in yeast cells exposed to UV light (80 J/m2) and incubated at 30 °C. As a control, one plate was not treated with UV radiation (0 J/m2). The assay was carried out at least three times, and data represent technical replicates from independent single yeast colonies. (c) Influence of Hsp90 variants on v-Src activation. Yeast cells expressing Hsp90 variants as the sole Hsp90 source and v-Src under the control of a galactose promoter were spotted on glucose (control, no v-Src expression)- and galactose-containing dropout plates (v-Src expression) and incubated at 30 °C. The assay was carried out at least three times, and data represent technical replicates started from independent single yeast colonies. (d) Influence of Hsp90 mutations on GR processing in yeast cells. GR activation, measured with a β-galactosidase-coupled assay, in yeast cells expressing different Hsp90 variants. Experiments were carried out at least three times, and data represent technical replicates (n = 3) started from independent single yeast colonies. Error bars, s.d. (e) Binding of the GR to different Hsp90 mutants, monitored by analytical ultracentrifugation with fluorescence detection and derived from dc/dt plots. GR* alone (brown; asterisk indicates labeled protein) or GR* in complex with WT (black), A107N (yellow), Δ8 (blue), T22I (green), R346S (purple), R380A (gray), E33A (red) and D79N (cyan) are shown. (f) Peak areas of the GR*–Hsp90 complex in the presence (gray bars) or absence of ATP (red bars).