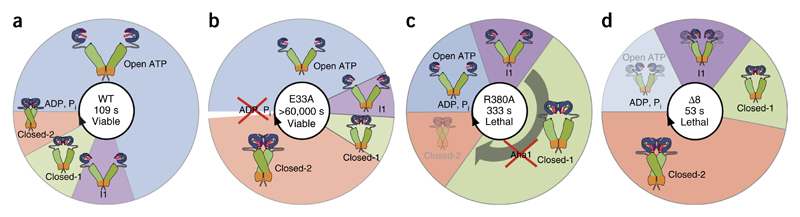
Figure 7.
Importance of cycle timing in the function of Hsp90. The scheme illustrates the differences in dwell times in different states (blue, open; violet, I1; green, closed-1; orange, closed-2) for WT Hsp90 (ref. 19) and mutants analyzed in this study. The sizes of the sectors indicate the relative time spent by the mutated protein in the different states during one round of hydrolysis. The average cycle time is indicated by the numbers in the centers of the circles. Mutants showing less intense effects, such as R364A, A107N and T22I, or those that did not bind nucleotide, such as D79N, are not depicted. (a) Hsp90 WT. (b) E33A. The white sector in the scheme for E33A indicates that hydrolysis did not detectably occur, and therefore the mutant does not appear to pass beyond this state. Release of ATP appears to rest the cycle. The variant shifts primarily between the open and the closed-2 state. (c) R380A. The conformational cycle of this mutant is dominated by closed states, especially closed-1. The defect cannot be corrected by Aha1. (d) Δ8. This mutant adopts a compact state even in the absence of nucleotide. It hydrolyzes ATP efficiently but populates primarily the closed-2 state.