Scheme 4.
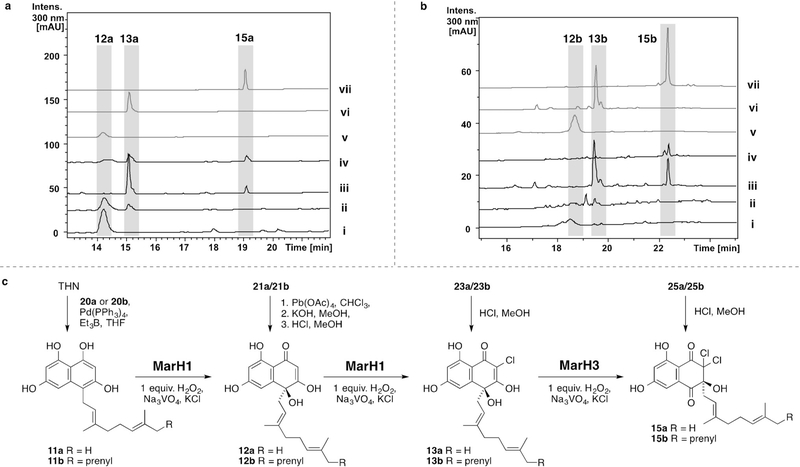
Reversed-phase HPLC chromatograms (300 nm) of MarH enzyme assays (i–iv) and comparison to synthetic standards (v–vii) with naphterpin (a) and marinone (b) substrates. i) 11a/11b, no enzyme, 2 equiv. H2O2; ii) 11a/11b, MarH1, 2 equiv. H2O2; iii) 13a/13b, MarH3, 1 equiv. H2O2; iv) 11a/11b, MarH1, and MarH3, 3 equiv. H2O2; v) 12a/12b; vi) 13a/13b; vii) 15a/15b. c) Reaction conditions for the chemical syntheses (vertical arrows) and MarH enzymatic conversions (horizontal arrows) of biosynthetic intermediates 11a/11b, 12a/12b, 13a/13b, and 15a/15b. Additional control experiments can be found in Figures S7–S26.