Figure 6.
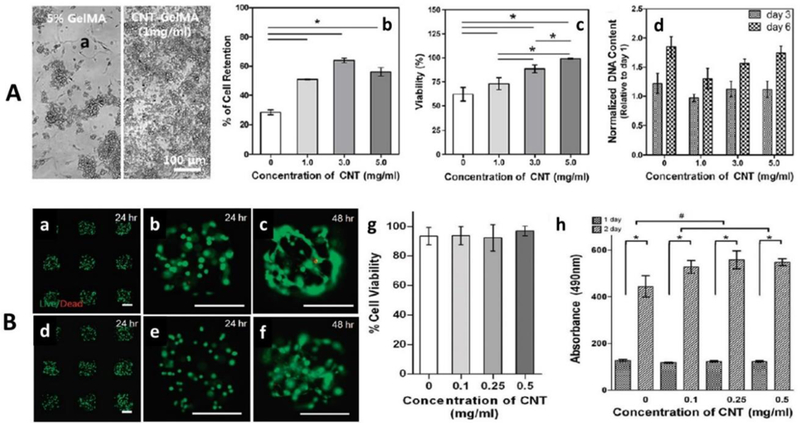
A) Biocompatibility assessment of CNTs-GelMA designed for cardiac construct a) morphology of GelMA and CNTs-GelMA in the presence of cell confirming good cell adhesion and cell retention, b) cell retention, c) cell viability, and normalized DNA quantities (at day 3 and 7) as a function of CNTs concentration (1 to 5 mg/mL).[53] B) Biocompatibility assessment of CNTs-GelMA system designed for ECM. a) Calcein-AM (green)/ethidium homodimer (red) Stained optical image of 3T3 fibroblasts cells embedded in GelMA (a to c) and micro-patterned CNTs-GelMA composite hydrogel (0.5 mg/mL) (d to f) for 24 and 48 h after encapsulation. The live/dead assay confirmed that developed system caused no toxicity, g) cell viability assay (after 24 h encapsulation) and h) MTS assay (absorption at 490 nm, after 24 and 48 h encapsulation) as a function of varying CNTs concentration.[106]
