ABSTRACT
Background
Protein-energy malnutrition (PEM) is a major problem in older adults. Whether poor diet quality is an indicator for the long-term development of PEM is unknown.
Objective
The aim was to determine whether poor diet quality is associated with the incidence of PEM in community-dwelling older adults.
Design
We used data on 2234 US community-dwelling older adults aged 70–79 y of the Health, Aging, and Body Composition (Health ABC) Study. In 1998–1999, dietary intake over the preceding year was measured by using a Block food-frequency questionnaire. Indicators of diet quality include the Healthy Eating Index (HEI), energy intake, and protein intake. Outcomes were determined annually by using measured weight and height and included the following: 1) incident PEM [body mass index (in kg/m2) <20, involuntary weight loss of ≥5% in the preceding year at any follow-up examination, or both] and 2) incident persistent PEM (having PEM at 2 consecutive follow-up examinations). Associations of indicators of diet quality with 4-y and 3-y incidence of PEM and persistent PEM, respectively, were examined by multivariable Cox regression analyses.
Results
The quality of the diet, as assessed with the HEI, was rated as “poor” for 6.4% and as “needs improvement” for 73.0% of the participants. During follow-up, 24.9% of the participants developed PEM and 8.5% developed persistent PEM. A poor HEI score was not associated with incident PEM or persistent PEM. Lower baseline energy intake was associated with a lower incidence of PEM (HR per 100-kcal/d lower intake: 0.98; 95% CI: 0.97, 0.99) and persistent PEM (HR: 0.97; 95% CI: 0.95, 0.99), although lower baseline protein intake was observed to be associated with a higher incidence of persistent PEM (HR per 10-g/d lower intake: 1.15; 95% CI: 1.03, 1.29).
Conclusions
These findings do not indicate that a poor diet quality is a risk factor for the long-term development of PEM in community-dwelling older adults, although there is an indication that lower protein intake is associated with higher PEM risk.
Keywords: energy intake, protein intake, Healthy Eating Index, undernutrition, aged, cohort study
INTRODUCTION
Protein-energy malnutrition (PEM) is the progressive loss of both lean body mass and adipose tissue resulting from insufficient consumption of protein and energy (1). Older adults are particularly vulnerable to development of PEM. For example, the prevalence of PEM, defined as a BMI (in kg/m2) <20, unintentional weight loss of ≥5% in the past 6 mo, or both, was observed to be 12% in Dutch community-dwelling older adults (≥65 y) with home care, 7% in those without home care, and 18% in those who were hospitalized or institutionalized (2). Although the relative number of older adults with PEM is lowest in community-dwelling older adults, the absolute number is highest, because 90–97% of older adults live at home (3, 4). Given the aging world population (5), the number of older adults suffering from PEM is expected to increase, which will likely increase rates of morbidity (6), loss of independence (7), and mortality (8–11) and will lead to higher health care use (11) and societal costs (12).
The diet of a large number of community-dwelling older adults is of insufficient quality (13). Previous research showed that the quality of the diet of community-dwelling older adults, as assessed by the Healthy Eating Index (HEI), could be rated as “poor” for 3–24% and as “needs improvement” for 68–75% (14–17). Furthermore, it is generally known that one of the main risk factors for the short-term development of PEM is a negative energy balance due to a low energy and protein intake (1), which is very common in older adults (18). Social, health, and psychological factors that reduce or alter food intake are likely responsible for these lower intakes and thereby indirectly contribute to the development of PEM (19–21). Because diet quality is a potentially modifiable risk factor for PEM, it may be an important target for its prevention.
Although a close link might be expected between poor diet quality and short-term development of PEM, it is less obvious whether poor diet quality predisposes the development of PEM over years. Research into the association between poor diet quality and PEM is scarce. Studies that investigated associations between dietary intake and PEM (22–24), all with the use of the Mini Nutritional Assessment (Nestlé Nutrition Institute), were mainly cross-sectional (22, 23) and performed in hospitalized (22, 24) or institutionalized (23) older adults. Studies that investigated associations of a diet quality indicator (e.g., Diet Quality Index score) with low BMI or weight loss in community-dwelling older adults (25–27) were all cross-sectional and showed conflicting results. To our knowledge, whether poor diet quality is a risk factor for the long-term development of PEM in community-dwelling older adults has not been prospectively investigated. Therefore, the present study aims to determine associations of multiple indicators of diet quality with the long-term incidence of PEM in community-dwelling older adults.
METHODS
Study population
The Health, Aging, and Body Composition (Health ABC) Study is a longitudinal cohort study aiming to examine risk factors for functional decline and disability in well-functioning older people, particularly focusing on changes in body composition with age. The Health ABC Study cohort includes 3075 community-dwelling black and white men and women aged 70–79 y living in the United States. Participants were recruited from a random sample of white Medicare-eligible residents and all black Medicare-eligible residents in the metropolitan areas of Memphis, Tennessee, and Pittsburgh, Pennsylvania, between April 1997 and June 1998. Participants were eligible if they met the following criteria: reported not using special equipment to get around; reported no difficulties walking 0.25 mile, climbing up 10 steps without resting, and performing basic activities of daily living; were free of cancer in the past 3y; planned to remain in the geographic area for ≥3 y; and were not enrolled in lifestyle intervention trials. All of the participants provided written informed consent. The study was approved by the institutional review boards of the University of Tennessee, Memphis, Tennessee, and the University of Pittsburgh, Pittsburgh, Pennsylvania.
Baseline measurements, comprising a home interview and a clinical examination, were performed in 1997–1998. Follow-up data were collected annually during a clinic visit, followed by a telephone interview after 6 mo. Dietary intake was assessed only at the first 12-mo follow-up examination of the Health ABC Study. Therefore, this examination served as the baseline for the present study.
Analytical sample
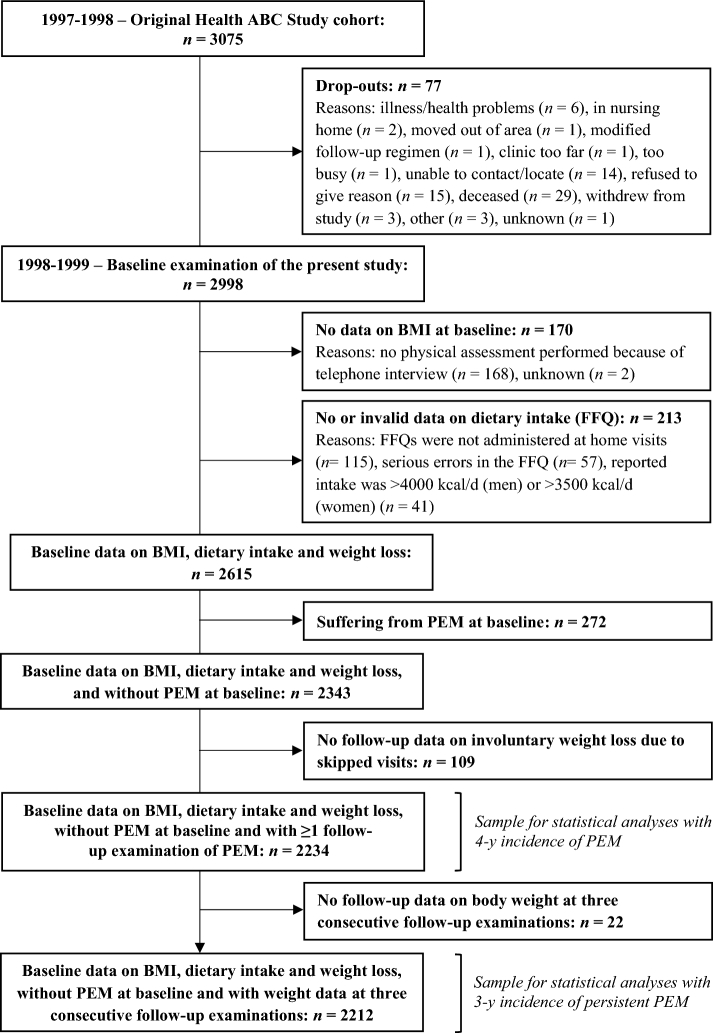
Participants were excluded if they dropped out before the baseline examination of the present study (n = 77), had no data on BMI at baseline (n = 170), had missing dietary data (n = 115), had serious errors on the food-frequency questionnaire (FFQ) (n = 57), reported high energy intakes (i.e., >3500 kcal/d for women or >4000 kcal/d for men) (n = 41), suffered from PEM at baseline (n = 272), or had no follow-up data on involuntary weight loss (n = 109). Finally, 2234 participants were included in the analyses with PEM. An additional 22 participants had to be excluded for the analyses with persistent PEM, because they had no data on body weight (BW) at 3 consecutive follow-up examinations, leaving an analytical sample of 2212 participants (Figure 1).
FIGURE 1.
Flowchart of participants included in the statistical analyses. FFQ, food-frequency questionnaire; Health ABC, Health, Aging, and Body Composition; PEM, protein-energy malnutrition.
Assessment of PEM and persistent PEM
During the annual clinical examination, standing height was measured to the nearest millimeter by using a Harpenden stadiometer (Holtain Ltd.). BW was measured to the nearest 0.1 kg by using a standard balance beam scale, with the participant wearing a standard clinic gown and without shoes or heavy jewelry. BMI was calculated by dividing measured BW (in kilograms) by measured height (in meters) squared. To minimize the influence of shrinking due to aging on BMI during follow-up, height measured at the Health ABC Study baseline examination was used in the calculation of BMI at all subsequent examinations. Weight loss in the past 12 mo was defined as the (relative) difference in measured BW between 2 consecutive annual follow-up examinations. Weight loss was considered unintentional if the participant reported the following: 1) not having tried to lose weight in the past 12 mo or 2) no loss of weight in the past 12 mo although substantial measured weight loss (i.e., ≥5%) was present. We defined PEM as a BMI (in kg/m2) <20, involuntary weight loss of ≥5% in the past 12 mo, or both (18). The BMI cutoff of 20 is commonly used for low BMI in older adults (18, 28–30), because optimal BMI is higher for older than for younger persons (20, 31, 32). Persistent PEM was defined as suffering from PEM at 2 consecutive follow-up examinations (i.e., suffering from PEM in year X combined with either having a BMI at the following year equal to or below the BMI of year X, or deceased in the following year). BMI and involuntary weight loss were determined annually, enabling us to determine PEM and persistent PEM every year from baseline through the fourth and third follow-up visits, respectively.
Dietary assessment
Dietary intake over the preceding year was assessed by a 108-item, interviewer-administered modified version of the Block FFQ (Block Dietary Data Systems) (33) at baseline (1998–1999). The FFQ food list was specially developed for the Health ABC Study on the basis of 24-h recall data from the NHANES III for older (>65 y) black and white adults living in the Northeast or the South. Interviewers were trained and used wood blocks, food models, standard kitchen measures, and flash cards to assist the participants in estimating their usual portion sizes accurately. Intakes of energy, macronutrients, and food groups were calculated by Block Dietary Data Systems (Berkeley, California). We examined multiple indicators of diet quality: the HEI, energy intake, and protein intake. For the present study, we used the 10-component HEI of 1994–1996 (34, 35), a score that reflects a participant's compliance with the Dietary Guidelines for Americans of 1995 (36) and the Food Guide Pyramid of 1992 (37). Five components measure to what degree the participant’s diet meets the age- and sex-specific serving recommendations, as specified in the Food Guide Pyramid, for 5 food groups: grains, vegetables, fruit, milk, and meat. Four components measure to what degree the participant's diet conforms to the dietary guidelines for intakes of total fat and saturated fat as percentages of total energy intake, total cholesterol, and total sodium. The last component measures the variety in the diet. Each component is assigned a score of 0 to 10, resulting in a sum score (the HEI) ranging from 0 to 100. A higher score reflects better diet quality. The HEI was categorized as good (score: >80), needs improvement (score: 51–80), and poor (score: <51) (34). Energy intake was expressed in kcal/d. Protein intake was expressed in g/d and in g ⋅ kg BW−1 ⋅ d−1. We used adjusted BW instead of actual BW to determine protein intake in grams per kilogram of BW per day. Adjusted BW is the nearest BW that would place the participants with an undesirable BW in the healthy BMI range of 18.5–24.9 for adults aged ≤70 y and of 22.0–27.0 for adults aged ≥71 y (38). We applied adjusted BW, because in overweight and obese people, much “extra weight” is fat tissue, whereas underweight people require extra protein to build muscle tissue. For the sake of readability, we will mention “g ⋅ kg BW−1 ⋅ d−1” when we refer to “g ⋅ kg adjusted BW−1 ⋅ d−1.” We dichotomized protein intake in grams per kilogram of BW per day into low (<0.8) and high (≥0.8) on the basis of the current Recommended Dietary Allowance for protein of 0.8 g ⋅ kg BW−1 ⋅ d−1 (39).
Assessment of covariates
At the Health ABC Study baseline examination, data on sociodemographic, lifestyle, and health-related factors were collected by using an interviewer-administered questionnaire. These data were used in our analyses, except for the variables (i.e., age, living arrangement, physical activity, smoking status, appetite, biting or chewing difficulty, and health status) that were also assessed at the first 12-mo follow-up examination.
Self-reported age, sex, and race were used. Educational level was assessed by self-reported highest grade of school completed and categorized into low (i.e., less than high school), medium (i.e., high school graduation), and high (i.e., postsecondary education). Income included all family income from salary, social security, retirement, help from relatives, and rent from property in the last year and was divided into 5 categories ranging from <$10,000 to ≥$50,000 and unknown. Living arrangement was determined by the self-reported number of household members and categorized into living alone and living with others. In case the number of household members was missing, we used self-reported marital status. Married participants were assumed to live with others, whereas widowed, divorced, separated, or never married participants were assumed to live alone. Overweight was defined as a BMI (in kg/m2) between ≥25 and <30 and obesity as a BMI ≥30 (40). Physical activity was measured by using a modified leisure-time physical activity questionnaire (41) and categorized into inactive (<1000 kcal/wk of exercise activity and ≤2719 kcal/wk of total physical activity), lifestyle active (<1000 kcal/wk of exercise activity and >2719 kcal/wk of total physical activity) and exercise active (≥1000 kcal/wk of exercise activity) (41). Smoking status was determined by self-reported smoking of cigarettes, pipes, or cigars and categorized into current, never, and former smokers. Current alcohol consumption (yes or no) was defined as the consumption of any alcoholic beverage in the preceding 12 mo, as derived from the FFQ. Appetite was assessed by using the question, “In the past month, would you say that your appetite or desire to eat has been…?” and dichotomized into good (very good and good) and poor (moderate, poor, and very poor). Biting or chewing difficulty was assessed by self-reported trouble biting or chewing any kinds of foods, such as firm meat or apples, and dichotomized into having (always, often, or sometimes) and not having (seldom, never) difficulties. The history or presence of chronic diseases (yes or no) was determined by using self-reported information on a physician's diagnosis of cancer, diabetes, cardiovascular disease, chronic pulmonary disease, and osteoporosis. Participants who reported that they did not know whether or not they have (had) the disease were supposed to have (had) not. For these chronic diseases, we combined data from the baseline and 6- and 12-mo follow-up examinations, if data were available. Kidney function was expressed as the estimated glomerular filtration rate (eGFR), which was calculated by using the 4-item Modification of Diet in Renal Disease equation (42). Serum creatinine concentration, as derived from blood samples, and age from the Health ABC Study baseline examination were used. Cognitive function was determined by the Modified Mini-Mental State examination (43), which is an abbreviated version of the Mini-Mental State Examination. Compared to the Mini-Mental State Examination, the Modified Mini-Mental State better differentiates among people with different stages of dementia (including nondementia) and has a higher reliability and higher sensitivity in detecting dementia (43, 44). Depression (yes or no) was assessed by the Center for Epidemiological Studies–Depression scale, a 20-item self-reported questionnaire for measuring the level of depressive symptomatology in the general population (45). A score ranging from 0 to 60 can be obtained, with a score ≥16 indicating depression (46). General health status indicates the participant’s self-reported health and was dichotomized into good (excellent, very good, good) and poor (fair, poor).
Statistical analyses
Baseline participant characteristics are presented as means ± SDs, medians with IQRs, or frequencies [n (%)]. Significant differences in baseline characteristics between participants who developed PEM and those who did not were tested by using chi-square tests for dichotomous and categorical variables and independent-samples t tests for continuous variables. Missing data were present on 15 covariates, with the proportion of missing values ranging from 0.04% (chronic pulmonary disease and osteoporosis) to 0.73% (eGFR and depression). The associations of indicators of diet quality with the incidence of PEM and the incidence of persistent PEM were examined by calculating HRs and 95% CIs by using multivariable Cox proportional hazards analyses. Follow-up time was defined, for PEM and persistent PEM separately, as the time between the baseline examination and 1) the first diagnosis of (persistent) PEM, 2) the last examination attended (e.g., due to loss to follow-up or death), or 3) the third (for persistent PEM) or fourth (for PEM) follow-up examination (end of study period), whichever came first.
In the present analyses, the HEI was analyzed categorically by using the category “good” as the reference. Energy intake was analyzed continuously and in quartiles by using the highest quartile (quartile 4) as the reference. Protein intake was analyzed continuously when expressed in g/d and dichotomously for low (<0.8) compared with high (≥0.8 g ⋅ kg BW−1 ⋅ d−1) protein intake. For continuous variables, we reversed the scale by multiplying all individual values by −1, to assess the potential risk of (persistent) PEM for lower intakes.
HRs were adjusted for potential confounders by building 2 sequential models. Model 1 was adjusted for age, sex, race, study site, educational level, income, living arrangement, physical activity, smoking status, appetite, biting or chewing difficulty, and energy intake (the latter was not adjusted for when energy intake was the determinant of interest). Model 2 was additionally adjusted for history or presence of cancer, diabetes, cardiovascular disease, chronic pulmonary disease and osteoporosis, eGFR, cognitive function, depression, and health status. Effect modification by age, sex, race, appetite, BMI, and physical activity was examined for each of the indicators of diet quality separately by adding a product term to the univariable Cox regression model (crude model). Because of multiple testing, an interaction was considered significant at P < 0.01. No significant interaction was observed.
The proportional hazards assumption was assessed by visual examination of log-log plots and by testing the interaction with time. No significant violations were observed. Statistical analyses were performed by using SPSS Statistics version 23 (IBM Corp.). Results were considered significant at P < 0.05 (2-sided).
In an additional analysis, we examined the associations of intakes of several food groups with incident PEM and incident persistent PEM. We examined 7 food groups: dairy; meat, fish, poultry, beans, and eggs; grains; fruit and fruit juices; vegetables; fats, oils, sweets, and sodas; and liquid supplements. Liquid supplements include meal supplements or replacements (e.g., Ensure; Abbott), dieting milkshakes (e.g., Slim-Fast; Kainos Capital), or instant breakfast milkshakes (e.g., Carnation; Nestlé Health Science). Intakes of food groups were analyzed continuously in servings per day and use of liquid supplements dichotomously [nonconsumers (0 kcal/d) compared with consumers (>0 kcal/d)]. Finally, in a sensitivity analysis, we examined associations of indicators of poor diet quality with 1-y incidence of PEM to find out whether the association between poor diet quality and incident PEM was different for short-term compared with long-term incidence of PEM.
RESULTS
At baseline, participants (n = 2234) were, on average, 74.6 ± 2.9 y old; 50.4% were women and 63.7% were white (Table 1). The participants who developed PEM during the 4-y follow-up (n = 557) were older and more often women and black than those who did not. Furthermore, participants who developed PEM more often lived alone, were less often current smokers, and reported a poorer appetite, more difficulties with biting or chewing food, and a poorer health status.
TABLE 1.
Baseline characteristics of the community-dwelling older adults of the Health ABC Study cohort, according to the development of PEM during 4 y of follow-up1
| Developed PEM during 4 y of follow-up2 | |||
|---|---|---|---|
| Total | No | Yes | |
| Participants, n | 2234 | 1677 | 557 |
| Age, y | 74.6 ± 2.93 | 74.5 ± 2.9 | 74.9 ± 2.8* |
| Female sex, n (%) | 1125 (50.4) | 813 (48.5) | 312 (56.0)# |
| White race, n (%) | 1424 (63.7) | 1103 (65.8) | 321 (57.6)# |
| Memphis study site, n (%) | 1085 (48.6) | 804 (47.9) | 281 (50.4) |
| Educational level, n (%) | |||
| Low: less than high school | 495 (22.2) | 357 (21.3) | 138 (24.9) |
| Medium: high school graduation | 733 (32.9) | 556 (33.2) | 177 (31.9) |
| High: postsecondary education | 1003 (45.0) | 763 (45.5) | 240 (43.2) |
| Income, n (%) | |||
| <$10,000 | 233 (10.4) | 156 (9.3) | 77 (13.8)# |
| ≥$10,000 to <$25,000 | 725 (32.5) | 538 (32.1) | 187 (33.6) |
| ≥$25,000 to <$50,000 | 659 (29.5) | 490 (29.2) | 169 (30.3) |
| ≥$50,000 | 357 (16.0) | 290 (17.3) | 67 (12.0) |
| Unknown (missing) | 260 (11.6) | 203 (12.1) | 57 (10.2) |
| Living alone, n (%) | 648 (29.1) | 465 (27.9) | 183 (32.9)* |
| BMI, kg/m2 | 27.8 ± 4.6 | 27.9 ± 4.6 | 27.3 ± 4.5* |
| Overweight (BMI: ≥25 and <30), n (%) | 986 (44.1) | 750 (44.7) | 236 (42.4) |
| Obese (BMI ≥30), n (%) | 585 (26.2) | 449 (26.8) | 136 (24.4) |
| Physical activity, n (%) | |||
| Inactive | 460 (20.6) | 328 (19.6) | 132 (23.7) |
| Lifestyle active | 1175 (52.7) | 884 (52.8) | 291 (52.3) |
| Exercise active | 595 (26.7) | 462 (27.6) | 133 (23.9) |
| Current smoker, n (%) | 168 (7.5) | 108 (6.4) | 60 (10.8)# |
| Current alcohol drinker, n (%) | 822 (36.8) | 628 (37.4) | 194 (34.8) |
| Poor appetite, n (%) | 441 (19.8) | 294 (17.6) | 147 (26.5)$ |
| Biting or chewing difficulty, n (%) | 454 (20.4) | 300 (18.0) | 154 (27.7)$ |
| Diseases (ever had), n (%) | |||
| Cancer | 485 (21.7) | 365 (21.8) | 120 (21.6) |
| Diabetes | 358 (16.0) | 265 (15.8) | 93 (16.7) |
| Cardiovascular disease | 646 (29.0) | 466 (27.9) | 180 (32.4)* |
| Pulmonary disease | 398 (17.8) | 288 (17.2) | 110 (19.7) |
| Osteoporosis | 191 (8.6) | 135 (8.1) | 56 (10.1) |
| eGFR | 72.5 ± 15.6 | 72.8 ± 15.2 | 71.5 ± 16.5 |
| Cognitive function, 3MS score | 91 ± 7 | 91 ± 7 | 90 ± 8* |
| Depression (CES-D score ≥16), n (%) | 99 (4.5) | 82 (4.9) | 17 (3.1) |
| Good general health status, n (%) | 1928 (86.4) | 1473 (87.9) | 455 (81.7)$ |
1Significant differences were estimated by using chi-square tests (dichotomous and categorical variables) and independent-samples t tests (continuous variables). *P < 0.05; #P < 0.01; $P < 0.001. CES-D, Center for Epidemiological Studies–Depression Scale; eGFR, estimated glomerular filtration rate; Health ABC, Health, Aging, and Body Composition; PEM, protein-energy malnutrition; 3MS, Modified Mini-Mental State Examination.
2Participants who developed PEM during the 4 y of follow-up and were free of PEM at baseline.
3Values are mean ± SD (all such values).
The quality of the diet, as assessed with the HEI, was rated as “needs improvement” for 73.0% of the participants and as “poor” for 6.4% (Table 2). A low protein intake (<0.8 g ⋅ kg BW−1 ⋅ d−1) was observed in 40.0% of the participants. The participants who developed PEM during the 4-y follow-up had, on average, similar HEI scores and energy intakes, and less often a low protein intake (<0.8 g ⋅ kg BW−1 ⋅ d−1) than did those who did not develop PEM.
TABLE 2.
Baseline diet quality of the community-dwelling older adults of the Health ABC Study cohort, according to the development of PEM during 4 y of follow-up1
| Developed PEM during 4 y of follow-up2 | |||
|---|---|---|---|
| Total | No | Yes | |
| Participants, n | 2234 | 1677 | 557 |
| Healthy Eating Index score, n (%) | |||
| Good (>80) | 460 (20.6) | 346 (20.6) | 114 (20.5) |
| Needs improvement (51–80) | 1631 (73.0) | 1233 (73.5) | 398 (71.5) |
| Poor (<51) | 143 (6.4) | 98 (5.8) | 45 (8.1) |
| Energy intake, kcal/d | 1829 ± 6393 | 1816 ± 634 | 1867 ± 653 |
| Energy intake in quartiles (kcal/d),4n (%) | |||
| Q1: 1122 (441–1356) | 558 (25.0) | 433 (25.8) | 125 (22.4) |
| Q2: 1557 (1356–1744) | 559 (25.0) | 418 (24.9) | 141 (25.3) |
| Q3: 1954 (1744–2211) | 559 (25.0) | 413 (24.6) | 146 (26.2) |
| Q4: 2610 (2211–3956) | 558 (25.0) | 413 (24.6) | 145 (26.0) |
| Protein intake, g/d | 65.2 ± 0.36 | 65.2 ± 25.0 | 66.6 ± 25.8 |
| Low protein intake,5n (%) | 894 (40.0) | 687 (41.0) | 207 (37.2) |
1Significant differences were estimated by using chi-square tests (dichotomous and categorical variables) and independent-samples t tests (continuous variables). *P < 0.05; #P < 0.01; $P < 0.001. Health ABC, Health, Aging, and Body Composition; PEM, protein-energy malnutrition; Q, quartile.
2Participants who developed PEM during the 4 y of follow-up and were free of PEM at baseline.
3Mean ± SD (all such values).
4Values are medians (minimum–maximum).
5Low protein intake: <0.8 g ⋅ kg adjusted body weight−1 ⋅ d−1.
During the 4-y follow-up (mean ± SD: 3.5 ± 0.9 y), 557 of the 2234 (24.9%) participants developed PEM. No association was observed between a poor HEI score and incident PEM, after adjustment for sociodemographic, lifestyle- and health-related factors, and energy intake (model 2) (Table 3). We observed that a 100-kcal/d lower energy intake was associated with a 2% lower incidence of PEM (HR: 0.98; 95% CI: 0.97, 0.99). No association was observed between any measure of protein intake and incident PEM. In addition, none of the food groups examined was observed to be associated with incident PEM (Supplemental Table 1).
TABLE 3.
HRs (95% CIs) for the associations of indicators of diet quality with 4-y incidence of PEM and 3-y incidence of persistent PEM in the well-nourished, community-dwelling older adults of the Health ABC Study cohort1
| Risk of developing PEM (4-y follow-up)2 | Risk of developing persistent PEM (3-y follow-up)3 | |||||
|---|---|---|---|---|---|---|
| Crude model | Model 14 | Model 25 | Crude model | Model 14 | Model 25 | |
| Healthy Eating Index score | ||||||
| Good (>80) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Needs improvement (51–80) | 0.99 (0.80, 1.22) | 0.91 (0.73, 1.13) | 0.93 (0.75, 1.16) | 1.09 (0.75, 1.59) | 0.94 (0.63, 1.38) | 0.95 (0.64, 1.41) |
| Poor (<51) | 1.32 (0.93, 1.87) | 1.11 (0.77, 1.60) | 1.15 (0.80, 1.66) | 1.30 (0.69, 2.46) | 0.92 (0.47, 1.81) | 0.94 (0.48, 1.85) |
| Energy intake per 100-kcal/d lower intake | 0.99 (0.97, 1.00)* | 0.98 (0.97, 0.99)# | 0.98 (0.97, 0.99)# | 0.98 (0.96, 1.00) | 0.97 (0.95, 1.00)* | 0.97 (0.95, 0.99)* |
| Energy intake in quartiles (kcal/d)6 | ||||||
| Q1: 1122 (441–1356) | 0.77 (0.60, 0.98)* | 0.72 (0.57, 0.93)* | 0.71 (0.55, 0.91)# | 0.63 (0.41, 0.96)* | 0.59 (0.38, 0.91)* | 0.56 (0.36, 0.87)* |
| Q2: 1557 (1356–1744) | 0.90 (0.71, 1.14) | 0.88 (0.69, 1.11) | 0.86 (0.67, 1.09) | 0.86 (0.58, 1.28) | 0.83 (0.55, 1.25) | 0.82 (0.54, 1.23) |
| Q3: 1954 (1744–2211) | 0.94 (0.75, 1.19) | 0.95 (0.75, 1.21) | 0.93 (0.73, 1.18) | 0.88 (0.59, 1.29) | 0.88 (0.59, 1.32) | 0.87 (0.59, 1.30) |
| Q4: 2610 (2211–3956) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Protein intake per 10-g/d lower intake | 0.98 (0.95, 1.01) | 0.99 (0.94, 1.05) | 1.00 (0.94, 1.06) | 1.01 (0.95, 1.07) | 1.14 (1.03, 1.28)* | 1.15 (1.03, 1.29)* |
| Low compared with high protein intake7 | 0.86 (0.72, 1.03) | 0.94 (0.75, 1.18) | 0.95 (0.76, 1.20) | 0.96 (0.71, 1.29) | 1.26 (0.85, 1.87) | 1.30 (0.87, 1.93) |
1HRs (95% CIs) were obtained from Cox proportional hazards analysis. *P < 0.05; #P < 0.01; $P < 0.001. Health ABC, Health, Aging, and Body Composition; PEM, protein-energy malnutrition; Q, quartile; ref, reference.
2 n cases/total n: 543/2166 (differs from original sample size due to missing covariates).
3 n cases/total n: 181/2135 (differs from original sample size due to missing covariates).
4Adjusted for age, sex, race, study site, educational level, income, living arrangement, physical activity, smoking status, appetite, biting or chewing difficulty, and energy intake. By using energy intake as the independent variable, models 1 and 2 were not additionally adjusted for energy intake.
5Additionally adjusted for history or presence of cancer, diabetes, cardiovascular disease, chronic pulmonary disease and osteoporosis, estimated glomerular filtration rate, cognitive function, depression, and health status.
6Values are medians (minimum–maximum).
7HRs reflect the association for low (<0.8 g ⋅ kg adjusted body weight−1 ⋅ d−1) compared with high (≥0.8 g ⋅ kg adjusted body weight−1 ⋅ d−1) protein intake.
Persistent PEM developed in 188 of the 2212 (8.5%) participants during the 3-y follow-up (mean ± SD: 2.7 ± 0.6 y). No association was observed between a poor HEI score and incident persistent PEM, after adjustment for confounders (Table 3). We observed that a 100-kcal/d lower energy intake was associated with a 3% lower incidence of persistent PEM (HR: 0.97; 95% CI: 0.95, 0.99). On the contrary, a lower absolute protein intake was associated with a higher incidence of persistent PEM (HR per 10-g/d lower intake: 1.15; 95% CI: 1.03, 1.29). A similar trend, although not significant, was observed for low (<0.8 g ⋅ kg BW−1 ⋅ d−1) compared with high (≥0.8 g ⋅ kg BW−1 ⋅ d−1) protein intake (HR: 1.30; 95% CI: 0.87, 1.93). No associations of intakes of food groups with incident persistent PEM were observed (Supplemental Table 1).
The use of 1-y incidence of PEM (144 of 2131 cases) instead of 4-y incidence of PEM did not markedly change the results; we only observed a significant association between energy intake and 1-y incidence of PEM (HR for a 100-kcal/d lower energy intake: 0.97; 95% CI: 0.95, 1.00; P < 0.05; HR for quartile 1 compared with quartile 4 of energy intake: 0.59; 95% CI: 0.36, 0.99; P < 0.05).
DISCUSSION
The present study in community-dwelling black and white older adults living in the United States showed that the majority of the participants consume a diet of insufficient quality. No associations of a poor HEI score (i.e., poor diet quality) with incident PEM or incident persistent PEM were observed. Unexpectedly, a lower baseline energy intake was associated with a lower incidence of PEM and persistent PEM, whereas there were some indications that a low protein intake was associated with a higher incidence of persistent PEM.
Comparison with other studies
To our knowledge, this is the first study that examined the association between a diet quality indicator and incident PEM in community-dwelling older adults. Our findings indicate no associations of a poor HEI score with incident PEM or persistent PEM. One explanation for our null findings might be that the HEI does not include the components that best fit older adults’ dietary requirements. The Dietary Guidelines for Americans, on which the HEI is based, were designed for the US population aged ≥2 y with the purpose to prevent chronic diseases (36, 47). The HEI is suitable for older adults as well because the guidelines for the 5 food groups contributing to the HEI are specified for different age and sex categories, including age ≥51 y (34). The HEI has also been linked to diseases and aging-related conditions in older adults (15, 48, 49). However, it is unknown whether the HEI is the most optimal index to assess the quality of older adults’ diet. Considering the rapidly growing older population, it seems worthwhile to develop a specific dietary index for older adults that also focuses on the prevention of malnutrition and functional decline. Such an index does not yet exist; the prevention of chronic diseases is the basis for almost all diet quality indicators (50, 51), although the components that make up these indexes differ and might therefore yield different results. This notion is supported by previous cross-sectional studies on diet quality and low BMI or weight loss in community-dwelling older adults. One study showed that a low BMI (<18.5) was associated with a lower Dietary Screening Tool score (i.e., poorer diet quality) in US men (aged ≥74 y) (26), whereas another study showed that a low BMI (<20.0) was associated with a higher modified Diet Quality Index–Revised score (i.e., better diet quality) in US men and women (aged ≥65 y) (27). In a study in German men and women (aged ≥75 y), a lower Mediterranean diet score (i.e., poorer diet quality) was associated with higher rates of self-reported weight loss of >4.5 kg in the past year (25). The diversity in the literature as well as our null findings for intakes of food groups preclude us from drawing conclusions on whether poor diet quality is a risk factor for the development of PEM.
This study showed that a lower energy intake was associated with a lower incidence of PEM and persistent PEM, regardless of the time of follow-up (1 or 4 y). This unexpected finding should be interpreted carefully, because it is illogical from a physiologic perspective that a higher energy intake would predispose to PEM, unless latent diseases would have had a major influence on the participant’s resting metabolic rate (52). However, because we adjusted our associations for a number of (chronic) diseases, we assume that any existing disease will not fully explain our findings. Nevertheless, different people have different energy requirements (regardless of any existing disease), but the absence of data on energy requirements precluded us from determining if a person's energy intake was really insufficient. However, additionally adjusting our associations for BW, body height, and physical activity did not alter the results. The possibility that participants underreported their energy intake should also be considered (53), but it is unclear how this could have affected our results. In sum, we cannot refuse the possibility that our finding is a chance finding and can neither confirm nor refute our hypothesis of energy intake being a risk factor for the long-term development of PEM.
The present study showed no associations of low (<0.8 g ⋅ kg BW−1 ⋅ d−1) compared with high (≥0.8 g ⋅ kg BW−1 ⋅ d−1) protein intake with incident PEM or persistent PEM. However, in an additional analysis, we observed that the HRs for low compared with high protein intakes were much higher (in absolute terms) when based on adjusted rather than on actual BW. The fully adjusted HRs (95% CIs) for low compared with high protein intakes on the basis of actual BW and adjusted BW were 0.79 (0.63, 0.98) and 0.95 (0.76, 1.20), respectively, for PEM, and 1.01 (0.69, 1.48) and 1.30 (0.87, 1.93), respectively, for persistent PEM. We expect these higher HRs for protein intake that is based on adjusted BW to be due to overweight and obese individuals because it is more difficult for them to reach the RDA of 0.8 g ⋅ kg BW−1 ⋅ d−1 if actual BW is used. We also observed that a lower absolute protein intake was associated with a higher incidence of persistent PEM, but not of PEM. Possibly, in the long term, protein intake is more strongly associated with a more chronic type of PEM. This idea is supported by a study that showed that a lower energy-adjusted protein intake is associated with a higher risk of lean mass loss in community-dwelling older adults (54). Therefore, although the evidence from our study is weak, a low protein intake might be a risk factor for the long-term development of (persistent) PEM.
Strengths and limitations
The present study has a number of strengths. First, we used measured BW (from each annual examination) and measured height (from baseline) to calculate (low) BMI and (involuntary) weight loss, so our study outcomes do not rely on self-reported height, weight, or weight loss, which are prone to bias (55). However, whether these are the best components to operationalize PEM is unknown due to the lack of consensus on the definition of PEM (56, 57) and its assessment, but we assume that these components are adequate because they are consistently used in the assessment of PEM (30, 56, 58). Furthermore, we explicitly did not use a screening tool, because such a tool is developed to screen persons with (risk of) PEM, after which an assessment should be performed to determine the presence of PEM (59). However, we acknowledge that other operationalizations of PEM exist, although they are not generally accepted (28, 60, 61). Second, the annual clinical examinations allowed us to determine (persistent) PEM regularly. Third, the fact that we did not observe an interaction with race enhances the generalizability of our findings to other populations. However, because our cohort included only black and white persons, we cannot ascertain that our findings apply to other races. Fourth, we conducted a prospective cohort study with a long follow-up (mean: 3.5 y). It might also be worth investigating if the findings would be similar if persons were followed from middle to old age; however, because the Health ABC Study included well-functioning older adults, their dietary habits are likely comparable to their habits in midlife. This study also has some limitations. Dietary intake, which was measured with the use of an FFQ, was assessed over the previous year, which has been shown to be difficult and may have led to inaccurate estimates of food intake (62). Furthermore, we cannot ignore the fact that the ability of an FFQ to estimate absolute dietary intake is limited (63, 64). We expect, however, that any arising misclassification was nondifferential, which may have attenuated the associations (65) and contributed to the null findings for a poor HEI score and intakes of food groups. In addition, an FFQ has been shown to be suitable in cohort studies for ranking individuals according to their average dietary intake, in order to relate diet to disease outcomes (63, 66, 67). We also cannot rule out the presence of residual confounding, but we expect this to have had little impact on our results because we adjusted for a wide range of confounders. Finally, the possibility of reverse causation has to be taken into account (68), although this seems very unlikely because of the long follow-up and the exclusion of malnourished cases at baseline.
Conclusions
The findings of the present study in US community-dwelling black and white older adults do not indicate that poor diet quality is a risk factor for the long-term development of PEM, although there is an indication that a higher protein intake is associated with a higher risk of persistent PEM. More prospective studies in community-dwelling older adults are warranted to replicate our study.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—LMH was involved in the design of the study, performed the statistical analyses, interpreted the data, wrote the initial manuscript, and revised the manuscript; HAHW, MRO, IAB, and MV: were involved in the design of the study, contributed to the interpretation of the data, and critically revised the manuscript; TBH, SBK and ABN: contributed to the interpretation of the data, and helped edit the manuscript; and all authors: read and approved the final manuscript. None of the authors declared a conflict of interest related to this research.
Notes
Supported in part by the Intramural Research Program of the NIH, National Institute on Aging (NIA) (NIA contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; National Institute of Nursing Research grant R01-NR012459). Funding for this article was also provided by the European Horizon 2020 PROMISS Project “PRevention Of Malnutrition In Senior Subjects in the EU” (grant agreement 678732). The content only reflects the authors’ view, and the European Commission is not responsible for any use that may be made of the information it contains.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used:
- BW
body weight
- eGFR
estimated glomerular filtration rate
- FFQ
food-frequency questionnaire
- Health ABC
Health, Aging, and Body Composition
- HEI
Healthy Eating Index
- PEM
protein-energy malnutrition.
REFERENCES
- 1. Chen CC, Schilling LS, Lyder CH. A concept analysis of malnutrition in the elderly. J Adv Nurs 2001;36:131–42. [DOI] [PubMed] [Google Scholar]
- 2. Undernutrition in the elderly The Hague (Netherlands): Health Council of the Netherlands; 2011. [Google Scholar]
- 3. Stula S. Living in old age in Europe: current developments and challenges. Berlin: Observatory for Sociopolitical Developments in Europe;2012. [Google Scholar]
- 4. Administration on Aging A profile of older Americans: 2015. US Department of Health and Human Services; 2015. [Cited 15 March 2017]. Available from: https://www.acl.gov/sites/default/files/Aging%20and%20Disability%20in%20America/2015-Profile.pdf. [Google Scholar]
- 5. He W, Goodkind D, Kowal P. An aging world: 2015. International population reports. Washington (DC): US Census Bureau; 2016. [Google Scholar]
- 6. Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 2003;22:235–9. [DOI] [PubMed] [Google Scholar]
- 7. Guest JF, Panca M, Baeyens JP, de Man F, Ljungqvist O, Pichard C, Wait S, Wilson L. Health economic impact of managing patients following a community-based diagnosis of malnutrition in the UK. Clin Nutr 2011;30:422–9. [DOI] [PubMed] [Google Scholar]
- 8. Cereda E, Pedrolli C, Zagami A, Vanotti A, Piffer S, Faliva M, Rondanelli M, Caccialanza R. Nutritional risk, functional status and mortality in newly institutionalised elderly. Br J Nutr 2013;110:1903–9. [DOI] [PubMed] [Google Scholar]
- 9. Gentile S, Lacroix O, Durand AC, Cretel E, Alazia M, Sambuc R, Bonin-Guillaume S. Malnutrition: a highly predictive risk factor of short-term mortality in elderly presenting to the emergency department. J Nutr Health Aging 2013;17:290–4. [DOI] [PubMed] [Google Scholar]
- 10. Kiesswetter E, Pohlhausen S, Uhlig K, Diekmann R, Lesser S, Uter W, Heseker H, Stehle P, Sieber CC, Volkert D. Prognostic differences of the Mini Nutritional Assessment short form and long form in relation to 1-year functional decline and mortality in community-dwelling older adults receiving home care. J Am Geriatr Soc 2014;62:512–7. [DOI] [PubMed] [Google Scholar]
- 11. Agarwal E, Ferguson M, Banks M, Batterham M, Bauer J, Capra S, Isenring E. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: results from the Nutrition Care Day Survey 2010. Clin Nutr 2013;32:737–45. [DOI] [PubMed] [Google Scholar]
- 12. Abizanda P, Sinclair A, Barcons N, Lizan L, Rodriguez-Manas L. Costs of malnutrition in institutionalized and community-dwelling older adults: a systematic review. J Am Med Dir Assoc 2016;17:17–23. [DOI] [PubMed] [Google Scholar]
- 13. USDA HEI-2010 total and component scores for children, adults, and older adults during 2011-2012. [Cited 14 December 2016]. Available from: https://www.cnpp.usda.gov/sites/default/files/healthy_eating_index/HEI-2010-During-2011-2012-Oct21-2016.pdf. [Google Scholar]
- 14. Ervin RB. Healthy Eating Index scores among adults, 60 years of age and over, by sociodemographic and health characteristics: United States, 1999–2002. Adv Data 2008:1–16. [PubMed] [Google Scholar]
- 15. Shahar DR, Yu B, Houston DK, Kritchevsky SB, Lee JS, Rubin SM, Sellmeyer DE, Tylavsky FA, Harris TB, for the Health ABC Study Dietary factors in relation to daily activity energy expenditure and mortality among older adults. J Nutr Health Aging 2009;13:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tangney CC, Evans DA, Bienias JL, Morris MC. Healthy eating index of black and white older adults. Nutr Res 2001;21:1411–23. [Google Scholar]
- 17. Vitolins MZ, Tooze JA, Golden SL, Arcury TA, Bell RA, Davis C, Devellis RF, Quandt SA. Older adults in the rural South are not meeting healthful eating guidelines. J Am Diet Assoc 2007;107:265–72. [DOI] [PubMed] [Google Scholar]
- 18. Nieuwenhuizen WF, Weenen H, Rigby P, Hetherington MM. Older adults and patients in need of nutritional support: review of current treatment options and factors influencing nutritional intake. Clin Nutr 2010;29:160–9. [DOI] [PubMed] [Google Scholar]
- 19. Elia M. Guidelines for detection and management of malnutrition. Maidenhead: Malnutrition Advisory Group, Standing Committee of BAPEN; 2000. [Google Scholar]
- 20. Soenen S, Chapman IM. Body weight, anorexia, and undernutrition in older people. J Am Med Dir Assoc 2013;14:642–8. [DOI] [PubMed] [Google Scholar]
- 21. Landi F, Calvani R, Tosato M, Martone AM, Ortolani E, Savera G, Sisto A, Marzetti E. Anorexia of aging: risk factors, consequences, and potential treatments. Nutrients 2016;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Oliveira MR, Leandro-Merhi VA. Food intake and nutritional status of hospitalised older people. Int J Older People Nurs 2011;6:196–200. [DOI] [PubMed] [Google Scholar]
- 23. Iuliano S, Poon S, Wang X, Bui M, Seeman E. Dairy food supplementation may reduce malnutrition risk in institutionalised elderly. Br J Nutr 2017;117:142–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soderstrom L, Rosenblad A, Adolfsson ET, Wolk A, Hakansson N, Bergkvist L. A high energy intake from dietary fat among middle-aged and older adults is associated with increased risk of malnutrition 10 years later. Br J Nutr 2015;114:915–23. [DOI] [PubMed] [Google Scholar]
- 25. Bollwein J, Diekmann R, Kaiser MJ, Bauer JM, Uter W, Sieber CC, Volkert D. Dietary quality is related to frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2013;68:483–9. [DOI] [PubMed] [Google Scholar]
- 26. Ford DW, Hartman TJ, Still C, Wood C, Mitchell D, Hsiao PY, Bailey R, Smiciklas-Wright H, Coffman DL, Jensen GL. Diet-related practices and BMI are associated with diet quality in older adults. Public Health Nutr 2014;17:1565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shannon J, Shikany JM, Barrett-Connor E, Marshall LM, Bunker CH, Chan JM, Stone KL, Orwoll E; Osteoporotic Fractures in Men Research Group Demographic factors associated with the diet quality of older US men: baseline data from the Osteoporotic Fractures in Men (MrOS) study. Public Health Nutr 2007;10:810–8. [DOI] [PubMed] [Google Scholar]
- 28. Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM, et al. Diagnostic criteria for malnutrition—an ESPEN Consensus Statement. Clin Nutr 2015;34:335–40. [DOI] [PubMed] [Google Scholar]
- 29. Edington J, Barnes R, Bryan F, Dupree E, Frost G, Hickson M, Lancaster J, Mongia S, Smith J, Torrance A, et al. A prospective randomised controlled trial of nutritional supplementation in malnourished elderly in the community: clinical and health economic outcomes. Clin Nutr 2004;23:195–204. [DOI] [PubMed] [Google Scholar]
- 30. Schilp J, Wijnhoven HA, Deeg DJ, Visser M. Early determinants for the development of undernutrition in an older general population: Longitudinal Aging Study Amsterdam. Br J Nutr 2011;106:708–17. [DOI] [PubMed] [Google Scholar]
- 31. Sergi G, Perissinotto E, Pisent C, Buja A, Maggi S, Coin A, Grigoletto F, Enzi G, for the ILSA working group An adequate threshold for body mass index to detect underweight condition in elderly persons: the Italian Longitudinal Study on Aging (ILSA). J Gerontol A Biol Sci Med Sci 2005;60:866–71. [DOI] [PubMed] [Google Scholar]
- 32. Winter JE, MacInnis RJ, Wattanapenpaiboon N, Nowson CA. BMI and all-cause mortality in older adults: a meta-analysis. Am J Clin Nutr 2014;99:875–90. [DOI] [PubMed] [Google Scholar]
- 33. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–69. [DOI] [PubMed] [Google Scholar]
- 34. Bowman SA, Lino M, Gerrior SA, Basiotis PP. The Healthy Eating Index: 1994–1996. Washington (DC): USDA, Center for Nutrition Policy and Promotion; 1998. [Google Scholar]
- 35. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc 1995;95:1103–8. [DOI] [PubMed] [Google Scholar]
- 36. USDA ; US Department of Health and Human Services Nutrition and your health: dietary guidelines for Americans. 4th ed Washington (DC): National Technical Information Service; 1995. [Google Scholar]
- 37. USDA, Center for Nutrition Policy and Promotion The Food Guide Pyramid. Washington (DC); 1992. [Cited 24 February 2017]. Available from: https://www.cnpp.usda.gov/sites/default/files/archived_projects/FGPPamphlet.pdf. [Google Scholar]
- 38. Berner LA, Becker G, Wise M, Doi J. Characterization of dietary protein among older adults in the United States: amount, animal sources, and meal patterns. J Acad Nutr Diet 2013;113:809–15. [DOI] [PubMed] [Google Scholar]
- 39. Institute of Medicine , Food and Nutrition Board. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington (DC): The National Academy Press; 2005. [Google Scholar]
- 40. Obesity: preventing and managing the global epidemic Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:i-xii, 1–253. [PubMed] [Google Scholar]
- 41. Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB; Health ABC Study The association between physical function and lifestyle activity and exercise in the Health, Aging and Body Composition Study. J Am Geriatr Soc 2004;52:502–9. [DOI] [PubMed] [Google Scholar]
- 42. National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–266. [PubMed] [Google Scholar]
- 43. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314–8. [PubMed] [Google Scholar]
- 44. Jones TG, Schinka JA, Vanderploeg RD, Small BJ, Graves AB, Mortimer JA. 3MS normative data for the elderly. Arch Clin Neuropsychol 2002;17:171–7. [PubMed] [Google Scholar]
- 45. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measur 1977;1:385–401. [Google Scholar]
- 46. Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): results from a community-based sample of older subjects in The Netherlands. Psychol Med 1997;27:231–5. [DOI] [PubMed] [Google Scholar]
- 47. USDA; US Department of Health and Human Services Dietary guidelines for Americans 2015–2020. 8th ed Washington (DC): USDA; US Department of Health and Human Services; 2015. [Google Scholar]
- 48. Koster A, Penninx BW, Newman AB, Visser M, van Gool CH, Harris TB, van Eijk JT, Kempen GI, Brach JS, Simonsick EM, et al. Lifestyle factors and incident mobility limitation in obese and non-obese older adults. Obesity (Silver Spring) 2007;15:3122–32. [DOI] [PubMed] [Google Scholar]
- 49. Xu B, Houston DK, Locher JL, Ellison KJ, Gropper S, Buys DR, Zizza CA. Higher Healthy Eating Index-2005 scores are associated with better physical performance. J Gerontol A Biol Sci Med Sci 2012;67:93–9. [DOI] [PubMed] [Google Scholar]
- 50. Gil A, Martinez de Victoria E, Olza J. Indicators for the evaluation of diet quality. Nutr Hosp 2015;31:128–44. [DOI] [PubMed] [Google Scholar]
- 51. Wirt A, Collins CE. Diet quality—what is it and does it matter? Public Health Nutr 2009;12:2473–92. [DOI] [PubMed] [Google Scholar]
- 52. Fabbri E, An Y, Schrack JA, Gonzalez-Freire M, Zoli M, Simonsick EM, Guralnik JM, Boyd CM, Studenski SA, Ferrucci L. Energy metabolism and the burden of multimorbidity in older adults: results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci 2015;70:1297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shahar DR, Yu B, Houston DK, Kritchevsky SB, Newman AB, Sellmeyer DE, Tylavsky FA, Lee JS, Harris TB, Health A, et al. Misreporting of energy intake in the elderly using doubly labeled water to measure total energy expenditure and weight change. J Am Coll Nutr 2010;29:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr 2008;87:150–5. [DOI] [PubMed] [Google Scholar]
- 55. Krul AJ, Daanen HA, Choi H. Self-reported and measured weight, height and body mass index (BMI) in Italy, the Netherlands and North America. Eur J Public Health 2011;21:414–9. [DOI] [PubMed] [Google Scholar]
- 56. Meijers JM, van Bokhorst-de van der Schueren MA, Schols JM, Soeters PB, Halfens RJ. Defining malnutrition: mission or mission impossible? Nutrition 2010;26:432–40. [DOI] [PubMed] [Google Scholar]
- 57. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet 2012;112:730–8. [DOI] [PubMed] [Google Scholar]
- 58. Neyens J, Halfens R, Spreeuwenberg M, Meijers J, Luiking Y, Verlaan G, Schols J. Malnutrition is associated with an increased risk of falls and impaired activity in elderly patients in Dutch residential long-term care (LTC): a cross-sectional study. Arch Gerontol Geriatr 2013;56:265–9. [DOI] [PubMed] [Google Scholar]
- 59. Charney P. Nutrition screening vs nutrition assessment: how do they differ? Nutr Clin Pract 2008;23:366–72. [DOI] [PubMed] [Google Scholar]
- 60. Mokaddem F. BMI and FFMI do not seem universally applicable in nutritional assessment and the usefulness of SGA and functional evaluation should not be overlooked. Clin Nutr 2016;35:236. [DOI] [PubMed] [Google Scholar]
- 61. Soeters P, Bozzetti F, Cynober L, Forbes A, Shenkin A, Sobotka L. Defining malnutrition: a plea to rethink. Clin Nutr 2017;36:896–901. [DOI] [PubMed] [Google Scholar]
- 62. Boyko EJ. Observational research—opportunities and limitations. J Diabetes Complications 2013;27:642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Biro G, Hulshof KF, Ovesen L, Amorim Cruz JA, for the EFCOSUM group Selection of methodology to assess food intake. Eur J Clin Nutr 2002;56:S25–32. [DOI] [PubMed] [Google Scholar]
- 64. Molag ML, de Vries JH, Ocke MC, Dagnelie PC, van den Brandt PA, Jansen MC, van Staveren WA, van't Veer P. Design characteristics of food frequency questionnaires in relation to their validity. Am J Epidemiol 2007;166:1468–78. [DOI] [PubMed] [Google Scholar]
- 65. Freedman LS, Schatzkin A, Midthune D, Kipnis V. Dealing with dietary measurement error in nutritional cohort studies. J Natl Cancer Inst 2011;103:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food-frequency questionnaires—a review. Public Health Nutr 2002;5:567–87. [DOI] [PubMed] [Google Scholar]
- 67. Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health 2014;36:e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sessler DI, Imrey PB. Clinical research methodology 1: study designs and methodologic sources of error. Anesth Analg 2015;121:1034–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.