Summary
Thymic function, determined by signal-joint/DβJβ T-cell rearrangement excision circle ratio, correlates with HIV disease progression. This work establishes the relevance of the thymus in HIV disease progression, helping to clarify the mechanisms underlying the rationale of early combination antiretroviral therapy onset.
Keywords: HIV disease progression, thymic function, sj/β-TREC ratio, LTNP, vertical infection.
Abstract
Background.
Thymic function has been mainly analyzed with surrogate peripheral markers affected by peripheral T-cell expansion, making it difficult to assess the role of thymic failure in human immunodeficiency virus (HIV) disease progression. The assay of signal-joint/DβJβ T-cell rearrangement excision circles (sj/β-TREC ratio) overcomes this limitation but has only been assayed in small cohorts. Thus, the aim of this study was to determine the role of thymic function, measured by the sj/β-TREC ratio, on CD4 T-cell maintenance in prospective HIV cohorts that include patients with a wide age range and different immunological phenotypes.
Methods.
Seven hundred seventy-four patients including typical progressors, long-term nonprogressors (LTNPs), and vertically HIV-infected subjects were analyzed. Thymic function was quantified in peripheral blood samples using the sj/β-TREC ratio. Associations between thymic function and CD4 T-cell dynamics and combination antiretroviral therapy (cART) onset were analyzed using linear, logistic, and Cox proportional hazard models.
Results.
Thymic function failure (sj/β-TREC ratio <10) was independently associated with HIV progression. In agreement, patients with distinctive high CD4 T-cell levels and low progression rates (vertically HIV-infected patients and LTNPs, including HIV controllers) had significantly higher thymic function levels whereas patients with thymic function failure had lower CD4 T-cell levels, lower nadir, and faster CD4 T-cell decay.
Conclusions.
This work establishes the relevance of thymic function, measured by sj/β-TREC ratio, in HIV disease progression by analyzing a large number of patients in 3 cohorts with different HIV disease progression phenotypes. These results support and help to understand the mechanisms underlying the rationale of early cART onset.
The thymus is the major organ involved in T-cell generation. Thymic involution, which peaks at puberty [1], leads to a decreased but heterogeneous thymic function during adulthood and old age [2]. The relevance of the thymus in T-cell regeneration became evident in severe lymphopenic scenarios as intensive chemotherapy and human immunodeficiency virus (HIV) infection [3, 4]. In this scenario we have previously reported that thymic function, as measured by thymic volume, is the best predictor of CD4 T-cell reconstitution in patients on highly active antiretroviral therapy [5, 6]. In addition, signal-joint T-cell rearrangement excision circles (sj-TRECs) and CD4+CD31+ cells, referred as thymic output and recent thymic emigrants, respectively, have been associated with HIV disease progression in adults [7] and perinatally infected patients [8].
However, these measurements are highly impacted by peripheral T-cell proliferation and the results derived from these techniques may reflect changes on T-cell proliferation and activation rather than the real impact of thymic function [9]. This limitation is especially relevant in diseases with a high reshaping of the peripheral T-cell pool, such as HIV infection. The signal-joint/DβJβ T-cell rearrangement excision circle (sj/β-TREC) ratio, when used as a surrogate marker of thymic function, has been shown to overcome these limitations [10–12]. Therefore, the impact of the thymic function, as measured by the sj/β-TREC ratio, in the maintenance of stable CD4 T-cell levels, is still unknown. For this reason we quantified the sj/β-TREC ratio in large cohorts including (1) middle-aged and elderly adults, who usually show a more advanced thymic involution; (2) vertically HIV-infected individuals; and (3) subjects with low rates of progression, as long-term nonprogressors (LTNPs).
Thus, the aim of this study was to determine the role of thymic function levels, assayed by sj/β-TREC ratio, on CD4 T-cell maintenance in diverse prospective cohorts including a wide age range and different immunological phenotypes associated with HIV disease progression.
METHODS
Patients and Cohorts
Peripheral blood samples were obtained from the Spanish HIV Hospital Gregorio Marañon (HGM) BioBank integrated in the Spanish AIDS Research Network (RIS) [13]; participant centers are shown in Supplementary Annex 1. Samples from a total of 774 donors were analyzed. Cohorts were as follows: (1) Progressors cohort: 483 patients were randomly selected from the cohort of adults with HIV infection of RIS (CoRIS), an open multicenter cohort of newly diagnosed patients naive to antiretroviral treatment at inclusion in the cohort. LTNPs and HIV controllers (see below) were excluded from this group. (2) LTNP cohort: 169 patients, defined exclusively based on immunological criteria, with CD4+ T-cell counts >500 cells/µL for >10 years after HIV diagnosis in the absence of antiretroviral treatment. Within this cohort, we identified 123 HIV controllers based on the following virological criteria: individuals who maintained, for at least 1 year in absence of antiretroviral treatment, plasma HIV loads between 50 and 2000 HIV-RNA copies/mL, “relative controllers” (RCs; n = 46); or undetectable viral load (<50 HIV-RNA copies/mL), “elite controllers” (ECs; n = 77). (3) Pediatric cohort: Samples from 122 vertically infected patients were obtained from the pediatric cohort (CoRISpe) fulfilling the inclusion criteria previously reported [14], in brief, individuals <18 years of age at diagnosis, with laboratory-confirmed HIV diagnosed, with at least 1 visit to the pediatrician since January 1995 and with predicted follow-up. Overall, the study was reviewed and approved by the scientific and ethic committee of the RIS Spanish HIV-HGM Biobank.
Thymic Function Quantification
The sj/β-TREC ratio [10–12] was analyzed from frozen peripheral whole blood samples, as a surrogated quantification marker of thymic function as previously reported [12]. In brief, 6 representative β-TRECs were quantified in the same multiplexed polymerase chain reaction (PCR) reaction and the sj-TREC in another PCR reaction. Both β- and sj-TREC amplicons from the first PCR round were reamplified together in a quantitative PCR assay using the LightCycler 480 system (Roche, Germany). Quantification was performed using highly sensitive specific probes as previously described [12]. Thymic function failure was considered when the sj/β-TREC ratio was <10, as previously reported [15].
Statistical Analysis
For linear and logistic regression tests, regression coefficient (β) or hazard ratio, respectively, and 95% confidence intervals were calculated first in a bivariate analysis for all explanatory variables, followed by a multivariate regression including all variables reaching a significant trend (P < .1). The relationship between time to event (CD4 T-cell count decline to <350 cells/µL and combination antiretroviral therapy [cART] onset) and thymic function failure was quantified using Kaplan-Meier curves and log rank-test bivariate analysis. Cox proportional hazards multivariate regression was performed in all variables reaching a significant level (P < .1) in the bivariate analysis. In all tests, a P value < .05 was considered to indicate statistical significance. All statistical procedures and graphs were performed using the IBM Statistical Package for the Social Sciences and GraphPad Prism software, respectively.
RESULTS
Characteristics of the Cohorts
As shown in Table 1, the progressor cohort had a median of 365 (interquartile range [IQR], 200–548) CD4 T-cells/µL and a nadir of 246 (IQR, 126–337) CD4 T-cells/µL. These patients were diagnosed for 0.4 (0.1–1.0) years, had a median age of 37 (IQR, 31–43) years, and had a median viral load of 4.5 (IQR, 3.7–5.1) log10 HIV-RNA copies/mL. In the LTNP cohort, after a median of 15 (IQR, 13–19) years since diagnosis, patients had a median of 721 (IQR, 552–966) CD4 T-cells/µL and were 42 (IQR, 39–42) years old at the sample collection. The pediatric cohort, composed of vertically infected patients, had a median of 14 (IQR, 9–17) years of age and infection, and 843 (IQR, 653–1166) CD4 T-cells/µL.
Table 1.
Characteristics of the Study Cohorts
| Characteristic | Progressors | LTNP | Pediatric |
|---|---|---|---|
| No. | 483 | 169 | 122 |
| Age, y, median (IQR) | 37 (31–43) | 42 (39–42) | 14 (9–17) |
| Age at diagnosis, y, median (IQR) | 35 (29–42) | 25 (22–31) | 0 |
| Sex (male) | 383 (79) | 111 (66) | 51 (42) |
| Transmission risk (sexual) | 389 (81) | 35 (21) | 0 |
| Time since diagnosis, y, median (IQR)a | 0.4 (0.1–1.0) | 15 (13–19) | 14 (9–17) |
| CD4 T-cell count, cells/µL, median (IQR) | 365 (200–548) | 721 (552–966) | 843 (653–1166) |
| CD4 nadir, cells/µL, median (IQR) | 246 (126–337) | 521 (390–657) | 418 (205–636) |
| Viral load, log10 HIV-RNA copies/mL, median (IQR) | 4.5 (3.7–5.1) | 2.0 (1.7–3.2) | 1.5 (1.3–1.7) |
| Naive for antiretroviral treatment | 337 (70) | 169 (100) | 0 (0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range; LTNP, long-term nonprogressor.
aTime since diagnosis is time since infection in the pediatric cohort.
Thymic Failure Is Independently Associated With CD4 T-Cell Maintenance and CD4 T-Cell Nadir in Viremic Progressors
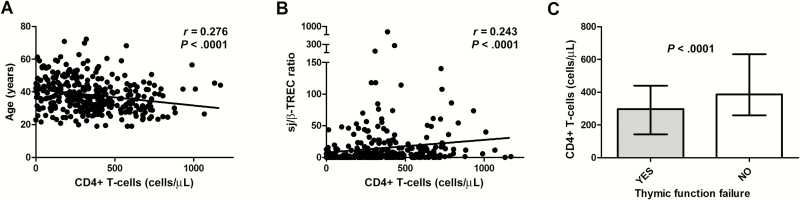
Thymic function, quantified by the sj/β-TREC ratio, was determined in 774 HIV-infected patients from the different cohorts. First, we analyzed all the patients (aged 1–75 years), to include different stages of thymic involution. Thymic function levels were overall associated with age (r = –0.354, P < .0001) and baseline CD4 T-cell level (r = 0.320, P < .0001). Afterward we selected progressor HIV-infected patients naive to antiretroviral treatment (viremic progressors, n = 337; Table 1). In these patients, CD4 T-cell levels were significantly associated with viral load (r = –0.324, P < .0001), age (Figure 1A), and thymic function (Figure 1B). Moreover, individuals with thymic function failure (sj/β-TREC ratio <10) had significantly lower CD4 T-cell levels (Figure 1C).
Figure 1.
Relationship between baseline CD4 T-cell numbers and age (A) and thymic function levels (B) in viremic progressors (n = 337); Spearman correlation. C, CD4 T-cell levels in patients with thymic function failure (thymic function failure threshold, signal-joint/DβJβ T-cell rearrangement excision circle (sj/β-TREC ratio <10); Mann-Whitney U test.
A bivariate regression analysis showed a significant association between CD4 T-cell levels and age, time since diagnosis, viral load, and thymic function, while age and viral load were the only variables independently associated in the multivariate analysis (Table 2). However, when thymic function failure was introduced as the categorical variable [15], age, time since diagnosis, viral load, and thymic function were independently associated with CD4 T-cell levels. In addition, thymic function and thymic function failure were independently associated with the CD4 T-cell nadir together with sex, age, and viral load (Table 2). These data strongly suggest that thymic failure has a direct impact in HIV progression in terms of CD4 T-cell level maintenance.
Table 2.
Linear Regression Analysis Between Baseline Variables and CD4 T-Cell Counts or CD4 T-Cell Nadir in Viremic Progressorsa (n = 337)
| Variable | Bivariate | Multivariateb | Multivariatec |
|---|---|---|---|
| P Value; β (95% CI)d | |||
| CD4 T-cell levels | |||
| Sex, male | .531; –19.1 (–79.0 to 40.8) | ||
| Age, y | <.001; –5.3 (–7.7 to –2.9) | <.001; –4.3 (–6.8 to –1.9) | .01; –3.9 (–6.3 to –1.5) |
| Time of diagnosis, y | .029; –9.0 (–17.1 to –0.9) | .082; –6.9 (–14.6 to –.9) | .01; –10.3 (–18.1 to –2.5) |
| Log10 VL, HIV-RNA copies/mL | <.001; –60.7 (–86.0 to –35.3) | <.001; –53.2 (–78.3 to –28.1) | <.001; –48.2 (–73.2 to –23.3) |
| sj/β-TREC ratio | .092; 0.4 (–.1 to .9) | .565; 0.14 (–.34 to .62) | |
| sj/β-TREC ratio <10e | <.001; –114.7 (–168.2 to –61.2) | .002; –81.9 (–134.5 to –29.2) | |
| CD4 nadir | |||
| Sex, male | .085; –29.1 (–62.3 to 4.0) | .011; –42.4 (–74.9 to –9.8) | .011; –41.5 (–73.5 to –9.4) |
| Age, y | <.001; –2.8 (–4.1 to –1.4) | .001; –2.3 (–3.7 to –.9) | .001; –2.2 (–3.6 to –.9) |
| Time since diagnosis, y | .400; –1.9 (–6.4 to 2.6) | ||
| Log10 VL, HIV-RNA copies/mL | <.001; –25.9 (–40.2 to –11.6) | .001; –23.6 (–37.8 to –9.5) | .003; –21.7 (–35.9 to –7.6) |
| sj/β-TREC ratio | .007; 0.4 (.1–.7) | .035; 0.3 (.02–.6) | |
| sj/β-TREC ratio <10e | <.001; –61.9 (–91.7 to –32.2) | .001; –48.9 (–19.2 to –78.5) | |
Dependent variables are shown underlined. Significant covariates are shown in bold. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; sj/β-TREC, signal-joint/DβJβ T-cell rearrangement excision circle; VL, viral load.
aNaive patients for antiretroviral treatment in the progressor cohort.
bAge, time of diagnosis, log10 VL, and sj/β-TREC ratio were used as covariates.
cAge, time of diagnosis, log10 VL, and sj/β-TREC ratio <10 were used as covariates.
dRegression coefficient and 95% confidence interval.
eThymic failure threshold.
Thymic Failure Is Associated With Faster CD4 T-Cell Loss and Early cART Onset
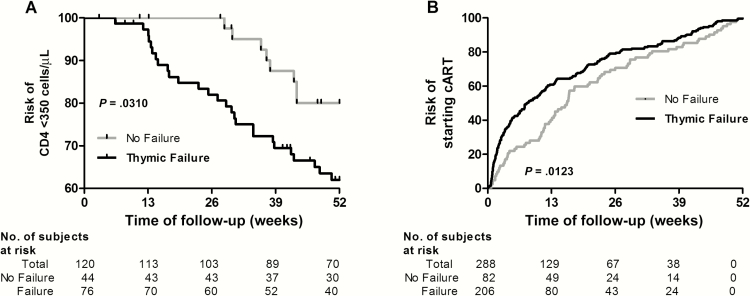
In another approach we first analyzed the risk of CD4 T-cell loss to reach 350 cells/µL, a common threshold to start cART. Individuals with baseline CD4 T-cell levels >350 cells/µL and >1 year of follow-up were selected from the viremic progressor group (120/337 [35.6%]) for this analysis. Fifty of 120 patients reached CD4 levels <350 cells/µL in 1 year. Despite the low number of events, individuals with thymic function failure at baseline showed a steeper CD4 decline after adjusting by baseline viral load, sex, age, and time since diagnosis. This association was not observed when thymic function was considered as a continuous variable (Figure 2A; Supplementary Table 1). Then we analyzed the time to the cART onset, independent of the CD4 T-cell levels. On the first year of follow-up, 288 patients started cART. Patients with thymic function failure at baseline started cART significantly earlier (Figure 2B). After adjusting for sex, age, time since diagnosis, and viral load, this association remained in a trend (Supplementary Table 2).
Figure 2.
Thymic failure, CD4 T-cell drop, and combination antiretroviral therapy (cART) onset. Kaplan-Meier curves and log-rank test for all time-to-event analyses for a 1-year follow-up. A, Risk of decreasing CD4 T-cell levels <350 cells/µL in patients with or without thymic failure at baseline. Only patients with baseline CD4 T-cell counts >350 cells/µL and at least 1 year of follow-up were included (n = 120). B, Risk of starting cART in patients with or without thymic failure at baseline (n = 288). Thymic failure threshold: signal-joint/DβJβ T-cell rearrangement excision circle ratio <10.
LTNPs and HIV Controllers Maintain Higher Thymic Function Levels Than Do Progressors
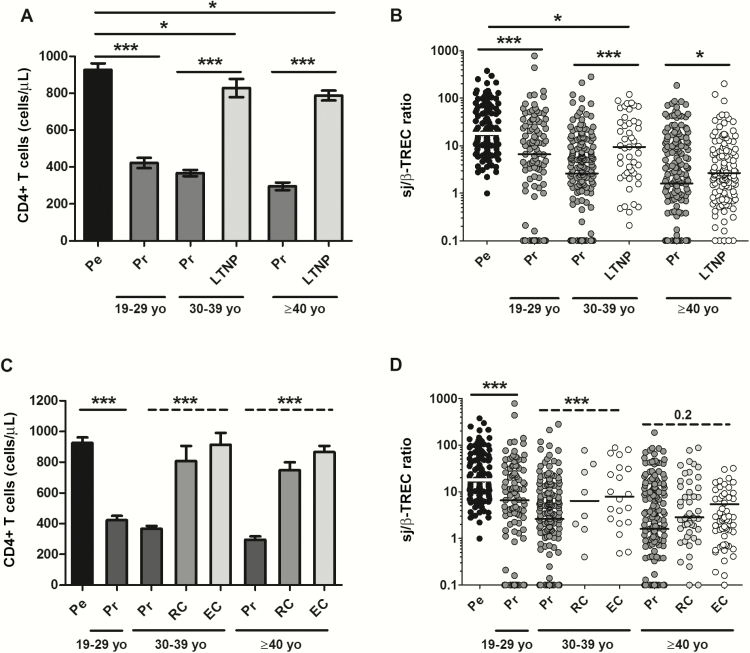
As expected, patients from the LTNP cohort had significantly higher CD4 T-cell counts than the general progressors cohort, and this difference was maintained after categorizing the patients into different age groups (Figure 3A). Thymic function was maximal in the pediatric cohort, and these levels were used as a reference [16] (Figure 3B). Within the progressors, thymic function was maximal in young adults (19–29 years of age) and significantly decreased with age (Figure 3B). LTNPs had higher thymic function than did progressors (Figure 3B), despite being older (P < .0001), and had been diagnosed for a longer time (P < .0001) (Table 1).
Figure 3.
Baseline CD4 T-cell (A) and thymic function (B) distribution among normal progressors (Pr; n = 483) and long-term nonprogressors (LTNP; n = 169). Patients from both groups are categorized into different age segments, including young adults (19–29 years old [yo]; n = 105 [16.1%]), middle-aged adults (age 30–39 years; n = 238 [36.5%]), and seniors (age ≥40 years; n = 309 [47.4%]). Pediatric samples (Pe; n = 122) are shown as a reference of high thymic function [14]. *P < .05, ***P < .0001; Mann-Whitney U test. CD4 T-cell (C) and thymic function (D) distribution among viremic patients (Vi; viral load [VL] >2000 copies/mL), relative controllers (RC; VL <2000 copies/mL), and elite controllers (EC; VL <50 copies/mL). Patients were categorized into different age segments. Pediatric samples (Pe) are shown as comparison. *P < .05, ***P < .0001, Mann-Whitney U test; ***P < .0001, analysis of variance nonparametric (Kruskal-Wallis test).
Next, to further study the role of thymic function in CD4 T-cell maintenance, we pooled together viremic progressor and LTNP cohorts and observed that thymic failure was independently associated with CD4 T-cell levels after adjusting by time since diagnosis, viral load levels, and inclusion or noninclusion in the LTNP cohort (Table 3).
Table 3.
Linear Regression Analysis of Factors Associated With CD4 T-Cell Counts in Viremic Progressorsa and Long-term Nonprogressorsb (n = 506)
| Variable | Bivariate | Multivariate |
|---|---|---|
| P Value; β (95% CI)c | ||
| Sex, male | .450; 25.6 (–40.9 to 92.1) | |
| Age, y | .833; 0.33 (–2.8 to 3.5) | |
| Time since diagnosis, y | <.0001; 22.8 (19.5–26.1) | .003; –9.9 (–16.3 to –3.4) |
| Log VL, HIV- RNA copies/ mL | <.0001; –145 (–162 to –128) | <.0001; –72.1 (–95.3 to –48.9) |
| Cohort, viremic progressors vs LTNPd | <.0001; 445 (397–493) | <.0001; 423 (308–538) |
| sj/β-TREC ratio <10e | .001; –107 (–171 to –43) | .001; –85 (–133 to –37) |
Significant covariates are shown in bold. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; LNTP, long-term nonprogressor; sj/β-TREC, signal-joint/DβJβ T-cell rearrangement excision circle; VL, viral load.
aNaive patients for antiretroviral treatment in the progressor cohort (n = 337).
bLong term-nonprogressors (n = 169).
cRegression coefficient and 95% confidence interval.
dCD4+ T-cell levels were adjusted by patient cohort (viremic progressors vs LTNP). LTNP cohort was taken as the reference. Previously we also included an interaction term ((Cohort) × sj/β-TREC ratio <10) ) that was finally excluded of the analysis because it was not statistically significant (.894; 6.88 [–94.0 to 107.7]).
eThymic failure threshold.
We then analyzed thymic function in a subgroup of LTNPs with spontaneous control of the viral load. The viremic progressors were now compared with RCs (n = 46) or ECs (n = 77). CD4 T-cell levels were similar between RCs and ECs, whereas progressors had significantly lower CD4 cell counts (Figure 3C). Following a similar trend, thymic function was similar between RCs and ECs, while thymic activity was always lower in progressors, independent of age group but especially in those 30–39 years old (Figure 3D). In the same way as with the whole LTNP cohort, pooling HIV controllers and viremic progressor cohorts, thymic failure was independently associated with CD4 T-cell levels after adjusting by time since diagnosis, viral load levels, and having or not having a controller profile (Supplementary Table 3).
Vertically HIV-Infected Patients Maintain the Highest Thymic Function Levels After Long-term Infection
Patients of the pediatric cohort (n = 122) showed the lowest frequency of thymic function failure (28.7% of patients with sj/β-TREC ratio <10, compared to 72% in progressors and 71.6% in LTNPs; P < .0001) despite being infected for a median of 14 (9–17) years (Table 1 and Figure 3B–D). In addition, thymic function in these patients was associated with CD4 T-cells levels after adjusting by viral load and age (Supplementary Table 4). Pooling together data from the 3 cohorts, only the age at diagnosis was independently associated with sj/β-TREC ratio levels (Supplementary Table 5). These results suggest that the age at infection is important to preserve thymic function. The age of diagnosis in the pediatric cohort was zero and, to avoid this bias, we repeated the analysis considering only the adult cohorts (progressors together with LTNPs). The results were reproducible in the cohort of adults, and age at diagnosis together with sex were the only variables independently associated with sj/β-TREC ratio levels (Supplementary Table 5).
DISCUSSION
Altogether, our results show that the thymic function has an active role in HIV progression by contributing to the maintenance of CD4 T-cell levels in different HIV-infected patients’ immunological phenotypes. In agreement, patients with distinctive high CD4 T-cell levels, as survivors infected by vertical transmission or with low progression rates for >10 years, have significantly higher thymic function levels compared with typical progressors naive for antiretroviral treatment.
In this work we present the largest collection of thymic function data in HIV-infected patients with a wide range of ages (1–75 years) by compiling sj/β-TREC ratio results from 3 prospective cohorts. We firmly associated this measurement of thymic function with different HIV disease-progression phenotypes. The sj/β-TREC ratio is, so far, the more accurate technique to measure thymic function [10]. The most used techniques to measure thymic output—the sj-TRECs [4, 17, 18] and CD31- and protein tyrosine kinase (PTK7)–naive T-cells are enriched in recent thymic emigrants (RTEs) [19]—are deeply affected by T-cell proliferation [9]. Peripheral T-cell proliferation and T-cell redistribution from tissues, especially in lymphopenic situations as HIV infection or aging, make difficult the interpretation of these techniques as accurate thymic function–related markers. Likewise, thymic volume measurement has the caveat of fat tissue infiltration, which makes difficult the discrimination of true thymic tissue and subsequent data analysis [5]. Hence, the sj/β-TREC ratio circumvents limitations of other works that have linked measurements associated with thymic function and HIV disease progression [7, 8].
Despite the fact that peripheral T-cell proliferation seems to contribute to the majority of naive T-cell levels in humans [20], this work shows the importance of thymic function in the maintenance of the CD4 T-cell pool in the lymphopenic scenario of HIV infection. We confirm that the thymic function failure threshold (sj/β-TREC ratio <10), which we showed to be independently associated with survival in elderly non-HIV-infected subjects [15], was a useful tool to categorize individuals with higher progression rates. In fact, thymic function failure was independently associated not only with CD4 T-cell levels, but also with the CD4 T-cell nadir in viremic progressors. These results may suggests that thymic function failure is a consequence of advanced disease and, although this analysis cannot assure causality, is more plausible that thymic function failure may establish the lowest CD4 T-cell level one individual can reach. Afterward, this threshold may predict the subsequent CD4 T-cell recovery after antiretroviral treatment onset [21]. In these sense, we have shown that baseline thymic function, as measured as thymic volume, predicts the magnitude of the CD4 T-cell reconstitution in the first year of highly active antiretroviral therapy [5, 6]. In fact, in the present study, thymic function failure was independently associated with CD4 T-cell decline, as well as a higher risk to start cART although, probably due to the heterogeneous incentives leading to the clinical decision for treatment onset, this association remained in a borderline statistical significance. All these results are in accordance with seminal works [7] showing the association of thymic output, measured by sj-TRECs, with HIV disease progression referred by time to AIDS and death in a seroconverter cohort of patients with hemophilia. However, the use of sj-TRECs in that work may be confounded or be parallel to T-cell activation and proliferation, which have also been factors associated with HIV disease progression [22].
Likewise, the role of thymic function in HIV progression is patent in the analysis of sj/β-TREC ratio in extreme phenotypes. In the larger dataset of thymic function analyzed in LTNPs to date, we observed that the ability of this rare group of subjects, able to naturally maintain normal CD4 T-cell levels (>500 cells/µL) for long periods of time even with active viral replication, was associated with higher thymic function. This result remained true for the subgroup of subjects able to spontaneously control viral replication, pointing out that high CD4 T-cell maintenance is independent of viral load levels. These results are in accordance with data in small cohorts of ECs [23] and LTNPs [11]. Hence, a supranormal thymopoiesis in LTNPs and HIV controllers, as a consequence of unknown genetic determinants [24], supports the production of naive T cells [11], which enables total CD4 T-cell maintenance and subsequently high CD4 T-cell nadir. This enhanced naive T-cell supply favors a more diverse and stable T-cell pool, perhaps accounting for the low rate of AIDS- and non-AIDS-defining events observed in these immunologically privileged patients [25]. Consistent with this hypothesis, HIV-infected patients with a low level of T-cell reconstitution have the lowest levels of thymic function [26, 27], lower CD4/CD8 ratio [28], and frequent nonAIDS-defining events [29].
This phenomenon of supranormal thymic function is even enhanced in subjects who had been infected by vertical transmission for a median of 14 years. This result is also in agreement with previous works showing that CD4+CD31+, as a measure of RTEs, is associated with preserved CD4 T-cell levels in a longitudinal prospective study [8] and even after 15 years of infection in cross-sectional designs [30]. In addition, a group of vertically infected patients showed similar thymic function levels as age-matched healthy controls after 20 years of infection [16]. However, as previously indicated, all these works have the caveat of putting at the same level CD31+ measurement with thymic function in conditions of heightened T-cell proliferation and activation, which makes it difficult to ascribe the weight of progression to thymic function based only on CD31+ measurement.
This high thymic function in vertically HIV-infected patients may be biased due to a selection process of patients included in this cohort (“survivors”; all samples were collected after 2005) in which individuals with lower thymic function die before or shortly after birth (“nonsurvivors”) and were not included. It may be also the case that this situation is the reflection of age at infection. This means that being infected at a younger age, as soon as birth, preserved the thymus from a higher damage. This later hypothesis is supported by the fact that, pooling together all the study cohorts but also restricting the analysis to the adult subgroup, the age at diagnosis is independently associated with thymic function. Interestingly, we found that thymic function is lower in males. This result needs further investigation but could be related to hormone levels as previous studies showed enhanced thymic function after sex steroid ablation [31].
This work has some limitations. The seroprevalent nature of the cohorts did not allow us to analyze thymic function during acute or early infection. Concerning the HIV controllers, all were extracted from the LTNP cohort, preventing us from analyzing whether thymic function failure is associated with low CD4+ T-cell levels in a subgroup of HIV controllers [23, 32]. Moreover, although all pediatric patients were treated with antiretrovirals at some point, we did not have the story and length of different treatments, which are known to affect thymic function in young subjects [33]; however, a reassuring result is that sj/β-TREC ratio was associated with CD4 T-cell levels even after adjusting by viral load.
In conclusion, this work definitely establishes the relevance of thymic function, measured by sj/β-TREC ratio, in HIV disease progression by the analysis of a large number of patients of 3 different cohorts with different progression phenotypes. These results greatly encourage the routine establishment of early cART onset in daily clinical practice and help to identify the biological mechanisms underlying the rationale for this procedure.
Supplementary Material
Notes
Acknowledgments. This study would not have been possible without the collaboration of all of the patients, medical and nursery staff, and data managers who have taken part in the project.
Financial support. This work was supported by Redes Tematicas de Investigacion en SIDA (ISCIII RETIC RD12/0017/0029 and RD06/0006/0035, RD12/0017/0037 and RIS-EPICLIN-08/2011); ISCIII Plataforma Red Nacional de BioBancos PT13/0010/0028, Proyecto de Excelencia, Consejeria de Innovacion, Ciencia y Empresa (P11-CTS-06313), Consejeria Andaluza de Salud (PI-0278-2010), Instituto de Salud Carlos III (CPII014/00025 to E. R.-M. and CPII13/00037 to Y. M. P.), PI12/02283 and PI16/00684 from Fondo de Investigación Sanitaria. Y.M. P. was supported also by the Consejería de Salud y Bienestar Social of Junta de Andalucía through the ‘‘Nicolás Monardes’’ program (C-0010/13).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Utsuyama M, Hirokawa K. Hypertrophy of the thymus and restoration of immune functions in mice and rats by gonadectomy. Mech Ageing Dev 1989; 47:175–85. [DOI] [PubMed] [Google Scholar]
- 2. Ferrando-Martínez S, Franco JM, Hernandez A, et al. Thymopoiesis in elderly human is associated with systemic inflammatory status. Age (Dordr) 2009; 31:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 1995; 332:143–9. [DOI] [PubMed] [Google Scholar]
- 4. Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998; 396:690–5. [DOI] [PubMed] [Google Scholar]
- 5. Ruiz-Mateos E, de la Rosa R, Soriano N, et al. Comparison of thymic function-related markers to predict early CD4 T-cell repopulation in adult HIV-infected patients on HAART. Antivir Ther 2003; 8:289–94. [PubMed] [Google Scholar]
- 6. Ruiz-Mateos E, Rubio A, Vallejo A, et al. Thymic volume is associated independently with the magnitude of short- and long-term repopulation of CD4+ T cells in HIV-infected adults after highly active antiretroviral therapy (HAART). Clin Exp Immunol 2004; 136:501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hatzakis A, Touloumi G, Karanicolas R, et al. Effect of recent thymic emigrants on progression of HIV-1 disease. Lancet 2000; 355:599–604. [DOI] [PubMed] [Google Scholar]
- 8. Zakhour R, Tran DQ, Degaffe G, et al. Recent thymus emigrant CD4+ T cells predict HIV disease progression in patients with perinatally acquired HIV. Clin Infect Dis 2016; 62:1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hazenberg MD, Otto SA, Cohen Stuart JW, et al. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T cell population in HIV-1 infection. Nat Med 2000; 6:1036–42. [DOI] [PubMed] [Google Scholar]
- 10. Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity 2004; 21:757–68. [DOI] [PubMed] [Google Scholar]
- 11. Dion ML, Bordi R, Zeidan J, et al. Slow disease progression and robust therapy-mediated CD4+ T-cell recovery are associated with efficient thymopoiesis during HIV-1 infection. Blood 2007; 109:2912–20. [DOI] [PubMed] [Google Scholar]
- 12. Ferrando-Martínez S, Franco JM, Ruiz-Mateos E, et al. A reliable and simplified sj/beta-TREC ratio quantification method for human thymic output measurement. J Immunol Methods 2010; 352(1–2):111–7. [DOI] [PubMed] [Google Scholar]
- 13. García-Merino I, de Las Cuevas N, Jiménez JL, et al. ; Spanish HIV BioBank The Spanish HIV BioBank: a model of cooperative HIV research. Retrovirology 2009; 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Jose MI, Jiménez de Ory S, Espiau M, et al. ; Working Groups of CoRISpe and HIV HGM BioBank A new tool for the paediatric HIV research: general data from the Cohort of the Spanish Paediatric HIV Network (CoRISpe). BMC Infect Dis 2013; 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrando-Martínez S, Romero-Sánchez MC, Solana R, et al. Thymic function failure and C-reactive protein levels are independent predictors of all-cause mortality in healthy elderly humans. Age (Dordr) 2013; 35:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aguilera-Sandoval CR, Yang OO, Jojic N, et al. Supranormal thymic output up to 2 decades after HIV-1 infection. AIDS 2016; 30:701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franco JM, Rubio A, Martínez-Moya M, et al. T-cell repopulation and thymic volume in HIV-1-infected adult patients after highly active antiretroviral therapy. Blood 2002; 99:3702–6. [DOI] [PubMed] [Google Scholar]
- 18. Zhang L, Lewin SR, Markowitz M, et al. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J Exp Med 1999; 190:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haines CJ, Giffon TD, Lu LS, et al. Human CD4+ T cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med 2009; 206:275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. den Braber I, Mugwagwa T, Vrisekoop N, et al. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity 2012; 36:288–97. [DOI] [PubMed] [Google Scholar]
- 21. D’Amico R, Yang Y, Mildvan D, et al. Lower CD4+ T lymphocyte nadirs may indicate limited immune reconstitution in HIV-1 infected individuals on potent antiretroviral therapy: analysis of immunophenotypic marker results of AACTG 5067. J Clin Immunol 2005; 25:106–15. [DOI] [PubMed] [Google Scholar]
- 22. Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 2003; 17:1881–8. [DOI] [PubMed] [Google Scholar]
- 23. Yang Y, Al-Mozaini M, Buzon MJ, et al. CD4 T-cell regeneration in HIV-1 elite controllers. AIDS 2012; 26:701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferrando-Martínez S, Ruiz-Mateos E, Dudakov JA, et al. WNT signaling suppression in the senescent human thymus. J Gerontol A Biol Sci Med Sci 2015; 70:273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dominguez-Molina B, Leon A, Rodriguez C, et al. ; Spanish AIDS Research Network HIV Controllers Cohort (ECRIS) Analysis of non-AIDS-defining events in HIV controllers. Clin Infect Dis 2016; 62:1304–9. [DOI] [PubMed] [Google Scholar]
- 26. Molina-Pinelo S, Vallejo A, Díaz L, et al. Premature immunosenescence in HIV-infected patients on highly active antiretroviral therapy with low-level CD4 T cell repopulation. J Antimicrob Chemother 2009; 64:579–88. [DOI] [PubMed] [Google Scholar]
- 27. Li T, Wu N, Dai Y, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV-infected patients after long-term antiretroviral therapy. Clin Infect Dis 2011; 53:944–51. [DOI] [PubMed] [Google Scholar]
- 28. Rosado-Sánchez I, Herrero-Fernández I, Genebat M, Ruiz-Mateos E, Leal M, Pacheco YM. Thymic function impacts the peripheral CD4/CD8 ratio of HIV-infected subjects. Clin Infect Dis 2017; 64:152–8. [DOI] [PubMed] [Google Scholar]
- 29. Pacheco YM, Jarrin I, Rosado I, et al. ; CoRIS Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res 2015; 117:69–74. [DOI] [PubMed] [Google Scholar]
- 30. Blanche S, Scott-Algara D, Le Chenadec J, et al. Naive T lymphocytes and recent thymic emigrants are associated with HIV-1 disease history in French adolescents and young adults infected in the perinatal period: the ANRS-EP38-IMMIP study. Clin Infect Dis 2014; 58:573–87. [DOI] [PubMed] [Google Scholar]
- 31. Sutherland JS, Goldberg GL, Hammett MV, et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol 2005; 175:2741–53. [DOI] [PubMed] [Google Scholar]
- 32. Dominguez-Molina B, Tarancon-Diez L, Hua S, et al. HLA-B*57 and IFNL4-related polymorphisms are associated with protection against HIV-1 disease progression in controllers [manuscript published online ahead of print 16 December 2016]. Clin Infect Dis 2016. pii:ciw833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandgaard KS, Lewis J, Adams S, Klein N, Callard R. Antiretroviral therapy increases thymic output in children with HIV. AIDS 2014; 28:209–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.