Abstract
Endometriosis, a common disorder affecting women of reproductive age, is characterized by ectopic growth of the endometrial tissues, altered steroid hormone response, and inflammation. Previous studies revealed that statins, selective inhibitors of the key step of mevalonate pathway, inhibit growth of endometrial stromal cells in vitro and reduce endometriotic lesions in murine models of endometriosis. This study evaluated the effects of simvastatin on the development of endometriosis in a baboon model of this disease. Sixteen baboons were randomly assigned to the treatment group (simvastatin, 20 mg daily) or to the control group. Endometriotic lesions were evaluated by laparoscopy after 3 months. The volume of red, orange-red, and white endometriotic lesions was significantly reduced by 78% in animals treated with simvastatin. The expression of a marker of proliferation, proliferating cell nuclear antigen (PCNA), was significantly reduced in animals receiving simvastatin in red lesions, white lesions, black lesions, and in adhesions. Simvastatin was also associated with an increase in the expression of estrogen receptor alpha in red lesions, and a decrease in the expression of estrogen receptor beta in black lesions, in adhesions, and in eutopic endometrium. Furthermore, simvastatin significantly reduced the expression of neopterin, a marker of inflammation, oxidative stress, and immune system activation. Collectively, the present findings indicate that the inhibition of the mevalonate pathway by simvastatin reduces the risk of developing endometriosis in the primate model of this disease by decreasing the growth of endometrial lesions, by modulating the expression of genes encoding for estrogen receptors, and by reducing inflammation.
Keywords: endometriosis, simvastatin, baboon, gene expression
Summary Sentence
In the baboon model of endometriosis, simvastatin reduced the volume of active lesions, altered gene expression. and reduced the serum level of neopterin, a marker of immune system activation in the baboon model of endometriosis.
Introduction
Endometriosis is defined as the presence of endometrial glandular and stromal cells outside the uterus. It is one of the most common and often devastating gynecologic disorders affecting up to 10% of women in reproductive age, and is associated with dysmenorrhea, dyspareunia, intermenstrual pain, and infertility [1–3]. While the pathophysiology of endometriosis is still not well understood, extensive evidence points to a key role of estrogens acting on eutopic and ectopic endometrial tissues via both estrogen receptor alpha (ESR1) and estrogen receptor beta (ESR2), altering the phenotype of these tissues and leading to increased cell proliferation, adhesiveness, and invasiveness [4–6]. Epigenetic studies demonstrate that endometriosis is associated with hypomethylation of the promotor region and the consequent upregulation of ESR2 [7]. Indeed, mRNA levels of Esr2 are greatly elevated in endometriotic tissues and an increased expression of ESR2 is thought to play a major role in the stimulation of cyclo-oxygenase (COX)2, the activation of the inflammasome, and ultimately the development of endometriotic lesions [5, 6, 8]. Increased COX2 is just one of many manifestations of local and systemic inflammation associated with endometriosis [9–13]. Another feature of endometriosis is an increase of oxidative stress manifested, for example, by the elevation of serum thiols and carbonyls, an increase of HSP70bB΄, as well as increased levels of oxidative and carbonyl stress markers [14–19]. Advanced endometriosis is associated with an increase of total oxidant status and oxidative stress index while total antioxidant status is reduced [20].
Despite the major impact of endometriosis on women's health and extensive research efforts, currently available medical treatments such as GnRH analogs, oral contraceptives, and progestins are often ineffective or are associated with significant side effects. Based on the above-discussed aspects of the pathophysiology of endometriosis, we proposed that statins may represent a novel treatment of this disorder. Statins are competitive inhibitors of the rate-limiting step of the mevalonate pathway; inhibition of this pathway leads to the decreased production of several biologically active downstream products, including cholesterol and substrates of isoprenylation (farnesyl pyrophosphate and geranyl-geranyl pyrophosphate) resulting in anti-proliferative and anti-inflammatory effects on many tissues [21–23]. We and other investigators reported that statins inhibit proliferation and promote apoptosis of eutopic and ectopic endometrial stromal tissues in vitro [24–28]. We also found that simvastatin decreased the invasiveness of endometrial stromal cells in cultures [29]. Studies in vivo demonstrated that statins are highly effective in the reduction of the number and size of endometriotic lesions in several murine models of endometriosis [30–32]. Statins also exerted anti-inflammatory effects by reducing the expression of monocyte chemotactic protein 1 in endometrial stromal cells in vitro and in endometriotic implants in a nude mouse model of endometriosis [33].
In view of these considerations, we embarked on a pilot study evaluating the effects of statin on a primate model of endometriosis. We chose a well-established baboon model to investigate the effects of simvastatin on endometriotic lesions in vivo, as well as the evaluation of expression of selected genes in ectopic and eutopic endometrial tissues. We also evaluated the serum level of neopterin, a marker of oxidative stress, inflammation, and the degree of immune system activation [34–36].
Materials and methods
Animals
Sixteen healthy adult female baboons, Papio Anubis, were used in the study. The animals were trapped in the wild, quarantined for 3 months, and handled as described previously [37, 38]. Animal care and all procedures in this study were carried out in accordance with the Institute of Primate Research standard operating procedures. The Institutional Scientific Evaluation and Review Committee and Animal Care and Use Committee of both the Institute of Primate Research and Yale University approved the study. Animals were randomly assigned to the control group (N = 8) and to simvastatin group (N = 8). Randomization of each animal was carried out by the laboratory technician (and not an animal attendant) by opening a sealed opaque envelope containing assignment to control or treatment group. Weights of animals were comparable: 12.3 ± 0.6 and 12.0 ± 0.7 kg in the control group and the simvastatin group, respectively (mean ± SEM).
Endometriosis was induced as described previously by seeding autologous endometrial tissues [37, 39]. Briefly, endometrial tissues were collected by uterine curettage on the first or second day of menses. The tissues were fragmented and the resulting paste (1000 mg ± 250 mg; mean ± SD) was seeded at laparoscopy in several peritoneal sites (uterosacral ligaments, uterovesical fossa, pouch of Douglas, ovaries, and ovarian fossa). Treatment with simvastatin (20 mg orally daily inserted into the banana meal) was initiated immediately after the laparoscopy and continued for 3 months. Animals in the control group underwent a second laparoscopy 6 weeks after the induction of endometriosis to assure establishment of disease. All animals in the control and simvastatin groups underwent laparoscopies 3 months after the induction of endometriosis; in each group, one animal was in follicular phase and the remaining seven animals were in mid-cycle to luteal-menstrual phases (based on observations of their perineum, i.e. perineal stage) [40]. A detailed inspection of peritoneal cavity was performed by the same team of observers and documented by videotaping. Endometriotic lesions were measured in three dimensions, and volume was calculated using the prolate ellipsoid formula (product of three dimensions multiplied by π/6). Endometriotic lesions were characterized and grouped into red lesions, red-orange (flame-like) lesions, white lesions, and black lesions. Adhesions were measured and described as filmy or dense. Blood samples were collected and serum was frozen. Following this laparoscopy, four of the eight animals in both the control group and in the simvastatin group were euthanized and necropsy was performed. Endometriotic lesions and eutopic endometrium samples were collected and stored frozen. Remaining animals in both groups were maintained without treatment for an additional 6 months and a repeat laparoscopy was performed; evaluations of peritoneal cavity were performed again; subsequently, the animals were euthanized and necropsies were performed.
Tissues
Endometriotic lesions and eutopic endometrial tissues were stored at –80°C. Samples were homogenized using the Qiagen (Valencia, CA) Tissuelyser and RNA/DNA/protein extractions were performed using the Qiagen Allprep system according to the manufacturer's instructions. cDNA synthesis was performed using the BioRad (Hercules, CA) iScript cDNA synthesis kit using 500 ng of RNA in each reaction as per the manufacturer's instructions. Primers (Table 1) were custom-designed using PerlPrimer and tested for primer dimers using melt curve analysis. All sample/primer pairs were run in triplicate using the BioRad CFX Connect qPCR system. Data were analyzed using the 2−ΔΔCt method using β-actin as the endogenous control. Data analysis was performed using Graphpad Prism (San Diego, CA).
Table 1.
Primer sequences.
| Forward | Reverse | |
|---|---|---|
| β-Actin | 5΄-GTGTGACGTGGACATCCGTA-3΄ | 5΄-GTACTTGCGCTCAGGAGGAG-3΄ |
| Esr1 | 5΄-TGGGAATGATGAAAGGTGGGA-3΄ | 5΄-CCCTGGTTCCTGTCCAAGAG-3΄ |
| Esr2 | 5΄-AGATTCCCGGCTTTGTGGAG-3΄ | 5΄-GAGCAGATGTTCCATGCCCT-3΄ |
| Pcna | 5΄-TCTGGAGGTGACGGGTTACT-3΄ | 5΄-CATCCTCGATCTTGGGAGCC-3΄ |
Determination of serum level of neopterin
Serum samples were collected at the time of laparoscopy from animals treated with simvastatin (N = 7) or from the control group (N = 7) and stored at –80°C until analysis. Serum samples from the remaining one animal in each group were degraded and were not suitable for analysis. Neopterin levels were evaluated at the baseline (at the time of induction of endometriosis), after 3 months of treatment, and at the time of termination of the study (6 months after completion of the treatment). Serum samples were analyzed in duplicate using the Genway Neopterin competitive ELISA kit as per the manufacture's specifications (Genway Biotech Inc., San Diego, CA). Absorbance was measured on the iMark Microplate reader (Bio-Rad, Hercules, CA), and a standard curve was calculated by four-parameter logistics using the Microplate Manager software. The absorbance of all samples fell within the minimum and maximum limits of the standard curve.
Statistical analysis
Data were analyzed using JMP pro 11 statistical software (SAS Institute, Cary). Data are presented as the mean ±SEM. Comparisons between the groups were performed using, as appropriate, the one-way or two-way analysis of variance, the Student t-test, or the Wilcoxon test. Distribution was assessed for normality using the Shapiro-Wilk test.
Results
Assessment of the number and size of endometriotic lesions
At baseline, during the induction of endometriosis, all animals were carefully evaluated for the presence of spontaneous endometriosis. Only one animal in the simvastatin group had a single black lesion measuring 1.5 by 1.5 by 0.5 mm. Table 2 summarizes the findings of laparoscopies performed 3 months after the induction of endometriosis. The numbers of all lesions in the control group and the simvastatin group were not statistically different. There was a trend toward lower combined volume of all lesions in the simvastatin group than in the control group, but it did not reach statistical significance (decrease by 69%; P = 0.08). Since red, orange-red, and white lesions are more likely to contain histologically confirmed endometriotic tissues than black lesions [41], these lesions were analyzed separately; the combined volume of these lesions was significantly lower in the simvastatin group than in the control group (by 78%; P = 0.04). Evaluations of adhesions indicated a trend toward smaller size of adhesions in the simvastatin group; however, this effect was not statistically significant. Four animals from the control and simvastatin groups were housed for additional 6 months following completion of 3-month treatment and final laparoscopies on the day of euthanasia were performed; at that time, both groups of animals did not significantly differ in the number, the type, or the volume of endometriotic lesions (Table 3).
Table 2.
Effect of simvastatin on the number and size of endometriotic lesions after 3 months.
| Variable | Control, N = 8 | Simvastatin, N = 8 | P-value |
|---|---|---|---|
| Number of all lesions | 12.1 ± 2.8 | 10.9 ± 2.5 | 0.74 |
| Combined number of red, orange-red, and white | 10.6 ± 2.4 | 7.6 ± 2.4 | 0.40 |
| Number of red lesions | 2.1 ± 1.1 | 0.6 ± 0.4 | 0.27 |
| Number of orange-red lesions | 1.4 ± 0.5 | 0.4 ± 0.3 | 0.11 |
| Number of white lesions | 7.1 ± 1.9 | 6.6 ± 1.4 | 0.84 |
| Number of black lesions | 1.5 ± 0.7 | 3.7 ± 1.3 | 0.25 |
| Volume of all lesions (mm3) | 121 ± 64 | 37 ± 9 | 0.08 |
| Combined volume of red, orange-red, and white lesions (mm3) | 117.4 ± 64.6 | 26.4 ± 6.9 | 0.04 |
| Volume of red lesions (mm3) | 8.7 ± 4.0 | 0.3 ± 0.2 | 0.17 |
| Volume of orange-red lesions (mm3) | 5.0 ± 2.5 | 0.4 ± 0.3 | 0.09 |
| Volume of white lesions (mm3) | 103.7 ± 66.3 | 25.7 ± 6.7 | 0.23 |
| Volume of black lesions (mm3) | 3.8 ± 1.5 | 10.3 ± 5.0 | 0.21 |
| Number of adhesions | 4.4 ± 1.0 | 3.8 ± 0.8 | 0.67 |
| Number of filmy adhesions | 1.1 ± 0.3 | 1.3 ± 0.5 | 1.00 |
| Number of dense adhesions | 3.3 ± 0.9 | 2.5 ± 0.4 | 0.63 |
| All adhesions (surface area, mm2) | 512 ± 214 | 172 ± 51 | 0.67 |
| All adhesions (length, mm) | 51.6 ± 15.8 | 15.2 ± 5.4 | 0.16 |
Each value represents mean ± SEM per animal; comparison between groups were performed using the Student t-test or the Wilcoxon test.
Table 3.
Endometriotic lesions and adhesions 6 months after discontinuation of simvastatin.
| Variable | Control N = 4 | Simvastatin N = 4 | P-value |
|---|---|---|---|
| Number of all lesions | 16.6 ± 4.3 | 11.5 ± 5.2 | 0.51 |
| Combined number of red, orange-red, and white | 10.0 ± 4.2 | 7.3 ± 3.5 | 0.64 |
| Number of red lesions | 1.0 ± 0.4 | 1.8 ± 1.8 | 0.69 |
| Number of orange-red lesions | 0 ± 0.0 | 0.3 ± 0.3 | 0.45 |
| Number of white lesions | 9.0 ± 4.1 | 5.3 ± 2.2 | 0.46 |
| Number of black lesions | 6.3 ± 1.1 | 4.3 ± 2.4 | 0.48 |
| Volume of all lesions (mm3) | 64.3 ± 28.1 | 47.9 ± 20.2 | 0.65 |
| Combined volume of red, orange-red, and white lesions (mm3) | 29.9 ± 20.2 | 35.3 ± 17.5 | 0.85 |
| Volume of red lesions (mm3) | 0.6 ± 0.3 | 1.6 ± 1.6 | 0.55 |
| Volume of orange-red lesions (mm3) | 0 ± 0.0 | 0.02 ± 0.02 | 0.36 |
| Volume of white lesions (mm3) | 29.3 ± 20.3 | 33.7 ± 16.9 | 0.87 |
| Volume of black lesions (mm3) | 34.4 ± 28.9 | 12.6 ± 4.6 | 0.48 |
| Number of adhesions | 4.0 ± 1.2 | 3.0 ± 0.7 | 0.51 |
| Number of filmy adhesions | 1.0 ± 0.4 | 0.8 ± 0.5 | 0.70 |
| Number of dense adhesions | 3.0 ± 1.1 | 2.3 ± 0.5 | 0.55 |
| All adhesions (surface area, mm2) | 356 ± 186 | 191 ± 86 | 0.45 |
| All adhesions (length, mm) | 38.3 ± 17.4 | 30.1 ± 7.3 | 0.70 |
Each value represents mean ± SEM per animal; comparison between groups were performed using the Student t-test or the Wilcoxon test.
Evaluation of tissues and serum
Assessment of mRNA expression of selected genes from endometriotic lesions is presented in Table 4. Since the stage of menstrual cycle may significantly alter expression of the above genes, this analysis was carried out only on specimens collected from animals in the luteal phase (four in each group). The expression of Pcna, a marker of proliferative activity, was significantly lower in the simvastatin group than in the control group in red lesions (by 62%; P = 0.005), in white lesions (by 58%; P = 0.001), in black lesions (by 61%; P = 0.001), and in adhesions (by 63%; P = 0.029).
Table 4.
Effect of simvastatin on the mRNA of selected genes in endometriotic lesions in a subset of animals in luteal phase.
| Variable | Na | Control | Simvastatin | P-value |
|---|---|---|---|---|
| PCNA | ||||
| Red lesions | 4 | 8.18 ± 1.05 | 3.46 ± 0.03 | 0.005* |
| White lesions | 4 | 9.96 ± 0.82 | 4.16 ± 0.33 | 0.001* |
| Black lesions | 4 | 10.14 ± 0.36 | 3.98 ± 0.98 | 0.001* |
| Adhesions | 4 | 9.17 ± 1.58 | 3.42 ± 1.26 | 0.029* |
| Eutopic endometrium | 4 | 8.28 ± 1.02 | 7.58 ± 1.10 | 0.41 |
| Esr1 | ||||
| Red lesions | 4 | 12.77 ± 0.51 | 16.35 ± 1.27 | 0.03* |
| White lesions | 4 | 8.94 ± 1.32 | 10.98 ± 1.71 | 0.38 |
| Black lesions | 4 | 13.11 ± 0.07 | 12.61 ± 1.11 | 0.68 |
| Adhesions | 4 | 11.24 ± 1.57 | 7.86 ± 1.20 | 0.13 |
| Eutopic endometrium | 4 | 9.52 ± 0.16 | 9.22 ± 0.32 | 0.45 |
| Esr2 | ||||
| Red lesions | 4 | 10.10 ± 1.72 | 10.50 ± 0.79 | 0.83 |
| White lesions | 4 | 5.96 ± 1.21 | 8.36 ± 1.17 | 0.20 |
| Black lesions | 4 | 11.72 ± 1.24 | 5.59 ± 0.29 | 0.003* |
| Adhesions | 4 | 8.01 ± 1.70 | 3.79 ± 0.46 | 0.02 |
| Eutopic endometrium | 4 | 5.49 ± 0.08 | 4.32 ± 0.07 | <0.001* |
| Esr1/Esr2 ratio | ||||
| Red lesions | 4 | 1.26 ± 0.47 | 1.56 ± 0.41 | 0.59 |
| White lesions | 4 | 1.50 ± 0.57 | 1.31 ± 0.46 | 0.80 |
| Black lesions | 4 | 1.12 ± 0.25 | 2.55 ± 0.52 | 0.09 |
| Adhesions | 4 | 1.40 ± 0.45 | 2.07 ± 0.59 | 0.40 |
| Eutopic endometrium | 4 | 1.73 ± 0.19 | 2.11 ± 0.32 | 0.34 |
Each value represents mean ± SEM. Lesions from four animals in each group were available.
aOnly one lesion of each type was evaluated per animal.
Since estrogens and estrogen receptors play an important role in the pathophysiology of endometriosis, the mRNA expression of Esr1 and Esr2 was also evaluated. The expression of Esr1 was significantly increased in red lesions of animals treated with simvastatin (by 28%; P = 0.03). In contrast, simvastatin treatment was associated with a significantly lower expression of Esr2 in black lesions (by 48%, P = 0.003), in adhesions (by 47%; P = 0.02), and in eutopic endometrium (by 21%, P < 0.001). However, these effects did not statistically significantly alter the ratio of Esr1 to Esr2.
The effect of simvastatin treatment on the serum level of neopterin is illustrated in Figure 1. At baseline, both groups had comparable serum neopterin levels. At the time of completion of 3-month treatment, animals in the simvastatin group had a significantly lower level of neopterin (by 43%, P < 0.05), while 6 months after discontinuation of treatment, animals in the simvastatin group had even lower neopterin level (by 53%, P < 0.001) than the animals in the control group.
Figure 1.
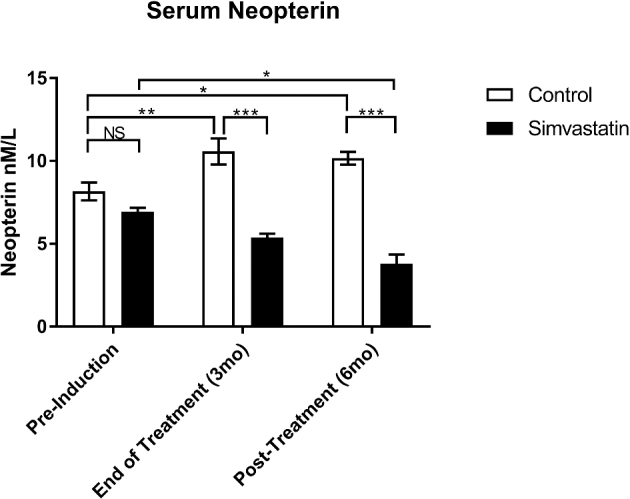
Effect of treatment on serum neopterin. Data analysis was performed using two-way analysis of variance followed by post-hoc pairwise comparisons. Neopterin level was determined from serum obtained on the day of induction of endometriosis (pre-induction), after 3 months of treatment (end of treatment) and 6 months after discontinuation of treatment (post-treatment).
Discussion
This report demonstrates for the first time that a statin affects endometriosis in a primate model of this disease. Specifically, we found that simvastatin treatment was associated with (i) a reduction in the combined volume of red, orange, and white endometriotic lesions; (ii) decreased expression of Pcna in several types of lesions; (iii) changes of Esr1 and Esr2 expression; and (iv) a decrease of serum neopterin.
The baboon model of endometriosis is well characterized, and it closely approximates many key features of human endometriosis including morphological appearance of lesions as well as alterations in the phenotype of endometriotic lesions including dysregulation of proteins involved in invasion, cell growth, and steroid hormone action [39, 42–44]. Present observations that the animals ingesting simvastatin had significantly lower volume of red, orange, and white lesions is consistent with several previous studies reporting that the statins simvastatin and atorvastatin greatly reduced endometriotic lesions in rat and mouse models of endometriosis [30–32]. This finding is also consistent with our studies of isolated human endometrial stromal cells, whereby statins reduced cell growth by inhibiting proliferation and promoting apoptosis [24, 28]. Indeed, in this study, in parallel with the decrease of the size of endometriotic lesions, simvastatin treatment was associated with a marked reduction of mRNA for Pcna in the majority of lesions/adhesions. PCNA is a well-recognized marker of proliferative activity of tissues [45] and a reduction of its expression in response to simvastatin has been demonstrated in several other biological systems [46–48]. However, it should be noted that antiproliferative effects of statins are not universal; thus, for example, simvastatin increased proliferation and expression of PCNA of human osteoblasts and alveolar epithelial cells [49, 50], demonstrating that these effects are tissue specific.
Other interesting findings in this study pertain to the pattern of expression of Esr1 and Esr2. While both ESR1 and ESR2 are important contributors to endometriosis, there is evidence for a decrease of ESR1 and an increase of ESR2 in endometriotic lesions in comparison to eutopic endometrium [5, 51].
In this context, it is interesting to note that in this study simvastatin exposure led to an altered expression of Esr1 and Esr2 in several endometrial lesions: an increase of Esr1 and a decrease of Esr2. Particularly relevant is the decreased expression of Esr2, since a recent groundbreaking study has demonstrated that ERß activates inflammatory responses by activating the inflammasome and increasing interleukin-1ß [6]. Endometriosis is characterized by inflammation, increased oxidative stress, and activation of the immune system [34–36]. In this context, it is interesting that we observed that the baboons exposed to simvastatin had significantly lower serum levels of neopterin than the control animals. Furthermore, neopterin levels continued to decline in the simvastatin group as observed 6 months after the discontinuation of simvastatin (Figure 1). Neopterin is a low-mass compound produced by activated human and other primate monocytes/macrophages in response to interferon gamma and other cytokines; concentrations of neopterin may be used as a marker of the extent of oxidative stress, inflammation, and the degree of immune system activation [34–36]. Serum levels of neopterin are significantly elevated in women with endometriosis [52].
Several limitations of this study should be discussed. First, this was a pilot study using only eight animals per treatment group. The use of such small number of animals was unavoidable due to logistical reasons, and hence the findings were affected by the variability inherent to the study of animals captured from the wild rather than inbred animals. Second, one has to be cautious while extrapolating the relevance of the observations from a baboon model of endometriosis to women with this disease. Third, there is no consensus regarding the “translation” of the dose of any drug across different species. One approach is to use the body surface area as a normalization method [53]. Using this method (human equivalent dose (mg/kg) = animal dose (mg/kg) × (Km baboon = 20/Km human = 37), the dose of simvastatin selected in this study (20 mg/day administered to a 12 kg baboon) would correspond to approximately 54 mg/day dose administered to a human weighing 60 kg. This dose is close to the dose of simvastatin (20–40 mg/day) typically administered to patients receiving long-term treatment for dyslipidemia. However, it should be noted that higher doses of simvastatin (e.g. 80 mg/day) are associated with an increased risk of myotoxicity compared with the maximum doses of other statins [54]. A significant limitation of this study was the performance of laparoscopy at 6 weeks after the induction of endometriosis in control animals only; this design was a result of using control animals also in another study. We acknowledge that performance of laparoscopy in control animals may have altered their immune response and may have affected the results. However, we noticed that the levels of neopterin, a marker of immune response, continued to decline in the simvastatin group after the second laparoscopy, as observed 6 months after the discontinuation of treatment (Figure 1). Another limitation of this study was the strict adherence to the schedule of a 3-month treatment and completion of the study irrespective of the cycle stage (perineal stage) of the animals. However, an alternative approach whereby the study would be completed at the identical stage of the cycle for each animal would result in marked differences in the duration of treatment. Finally, since the administration of simvastatin was commenced on the same day as induction of endometriosis, the present observations are pertinent to potential protection from growth of endometriotic lesions, for example, to reduce the recurrence of this disease after surgery, rather than treatment of established disease. The above limitations notwithstanding, the present observations encourage consideration for further studies of statins as novel therapeutic agents in endometriosis.
To date, little is known regarding the effects of statins on human endometriosis. One recent study evaluated pain recurrence after surgery for endometriosis and found that pain scores were comparable in subjects receiving simvastatin (20 mg/day) or Decapeptyl (3.75 mg every 4 weeks) [55]. It is not known, however, whether the use of statin(s) has an effect on human endometriotic lesions. Notably, there is a paucity of clinical trials evaluating the effects of statins in premenopausal women, largely due to concerns regarding potential teratogenicity of statins [56]; hence, the use of these medications should be restricted to women using reliable contraception. In women with polycystic ovary syndrome, who typically suffer from oligomenorrhea and hyperandrogenism, simvastatin improved menstrual cyclicity and reduced androgen levels [57]; however, the effects of statins on reproductive function of women with normal ovarian function are not known. In view of the above considerations, the routine use of statins in treatment of endometriosis cannot be recommended until rigorous clinical trials demonstrate both the efficacy and long-term safety.
In summary, our findings suggest that the inhibition of the mevalonate pathway using simvastatin reduces the burden of endometriosis in the baboon model of this disease. Potential mechanisms of action of simvastatin include a reduction of the growth of endometrial lesions, modulation of expression of genes encoding for estrogen receptors, and a reduction of systemic inflammation.
Acknowledgments
We acknowledge excellent assistance of the personnel of the Institute of Primate Research in Nairobi, Kenya.
Conflict of interest: Co-author Thomas D'Hooghe has been appointed as Vice-President and Head Global Medical Affairs Fertility at Merck, Darmstadt, Germany, since October 1st 2015. He was full time employed at the KU Leuven (University of Leuven) at the time of this study, and is partially appointed as Professor in Reproductive Medicine and Biology at KU Leuven (University of Leuven) since October 1, 2015.
References
- 1. Abbas S, Ihle P, Koster I, Schubert I. Prevalence and incidence of diagnosed endometriosis and risk of endometriosis in patients with endometriosis-related symptoms: findings from a statutory health insurance-based cohort in Germany. Eur J Obstet Gynecol Reprod Biol 2012; 160:79–83. [DOI] [PubMed] [Google Scholar]
- 2. Vercellini P, Vigano P, Somigliana E, Daguati R, Abbiati A, Fedele L. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstet Gynaecol 2006; 20:465–477. [DOI] [PubMed] [Google Scholar]
- 3. Vigano P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol 2004; 18:177–200. [DOI] [PubMed] [Google Scholar]
- 4. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab 2000; 85:2897–2902. [DOI] [PubMed] [Google Scholar]
- 5. Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H, Su EJ. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med 2012; 30:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han SJ, Jung SY, Wu SP, Hawkins SM, Park MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, DeMayo FJ, O'Malley BW. Estrogen Receptor beta Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015; 163:960–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Koukoura O, Sifakis S, Spandidos DA. DNA methylation in endometriosis (Review). Mol Med Rep 2016; 13:2939–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monsivais D, Dyson MT, Yin P, Coon JS, Navarro A, Feng G, Malpani SS, Ono M, Ercan CM, Wei JJ, Pavone ME, Su E et al. . ERbeta- and prostaglandin E2-regulated pathways integrate cell proliferation via Ras-like and estrogen-regulated growth inhibitor in endometriosis. Mol Endocrinol 2014; 28:1304–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arici A, Matalliotakis I, Goumenou A, Koumantakis G, Vassiliadis S, Mahutte NG. Altered expression of interleukin-18 in the peritoneal fluid of women with endometriosis. Fertil Steril 2003; 80:889–894. [DOI] [PubMed] [Google Scholar]
- 10. Tsudo T, Harada T, Iwabe T, Tanikawa M, Nagano Y, Ito M, Taniguchi F, Terakawa N. Altered gene expression and secretion of interleukin-6 in stromal cells derived from endometriotic tissues. Fertil Steril 2000; 73:205–211. [DOI] [PubMed] [Google Scholar]
- 11. Kao AP, Wang KH, Long CY, Chai CY, Tsai CF, Hsieh TH, Hsu CY, Chang CC, Lee JN, Tsai EM. Interleukin-1beta induces cyclooxygenase-2 expression and promotes the invasive ability of human mesenchymal stem cells derived from ovarian endometrioma. Fertil Steril 2011; 96:678–684.e671. [DOI] [PubMed] [Google Scholar]
- 12. Bertschi D, McKinnon BD, Evers J, Bersinger NA, Mueller MD. Enhanced inflammatory activity of endometriotic lesions from the rectovaginal septum. Mediators Inflamm 2013; 2013:450950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malutan AM, Drugan T, Costin N, Ciortea R, Bucuri C, Rada MP, Mihu D. Pro-inflammatory cytokines for evaluation of inflammatory status in endometriosis. Cent Eur J Immunol 2015; 40:96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lambrinoudaki IV, Augoulea A, Christodoulakos GE, Economou EV, Kaparos G, Kontoravdis A, Papadias C, Creatsas G. Measurable serum markers of oxidative stress response in women with endometriosis. Fertil Steril 2009; 91:46–50. [DOI] [PubMed] [Google Scholar]
- 15. Polak G, Wertel I, Barczynski B, Kwasniewski W, Bednarek W, Kotarski J. Increased levels of oxidative stress markers in the peritoneal fluid of women with endometriosis. Eur J Obstet Gynecol Reprod Biol 2013; 168:187–190. [DOI] [PubMed] [Google Scholar]
- 16. Di Emidio G, D'Alfonso A, Leocata P, Parisse V, Di Fonso A, Artini PG, Patacchiola F, Tatone C, Carta G. Increased levels of oxidative and carbonyl stress markers in normal ovarian cortex surrounding endometriotic cysts. Gynecol Endocrinol 2014; 30:808–812. [DOI] [PubMed] [Google Scholar]
- 17. Rosa e Silva JC, do Amara VF, Mendonca JL, Rosa e Silva AC, Nakao LS, Poli Neto OB, Ferriani RA. Serum markers of oxidative stress and endometriosis. Clin Exp Obstet Gynecol 2014; 41:371–374. [PubMed] [Google Scholar]
- 18. Santulli P, Chouzenoux S, Fiorese M, Marcellin L, Lemarechal H, Millischer AE, Batteux F, Borderie D, Chapron C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum Reprod 2015; 30:49–60. [DOI] [PubMed] [Google Scholar]
- 19. Tawadros PS, Powers KA, Ailenberg M, Birch SE, Marshall JC, Szaszi K, Kapus A, Rotstein OD. Oxidative stress increases surface Toll-like receptor 4 expression in murine macrophages via ceramide generation. Shock 2015; 44:157–165. [DOI] [PubMed] [Google Scholar]
- 20. Turgut A, Ozler A, Goruk NY, Tunc SY, Evliyaoglu O, Gul T. Copper, ceruloplasmin and oxidative stress in patients with advanced-stage endometriosis. Eur Rev Med Pharmacol Sci 2013; 17:1472–1478. [PubMed] [Google Scholar]
- 21. Ni W, Egashira K, Kataoka C, Kitamoto S, Koyanagi M, Inoue S, Takeshita A. Antiinflammatory and antiarteriosclerotic actions of HMG-CoA reductase inhibitors in a rat model of chronic inhibition of nitric oxide synthesis. Circ Res 2001; 89:415–421. [DOI] [PubMed] [Google Scholar]
- 22. Dichtl W, Dulak J, Frick M, Alber HF, Schwarzacher SP, Ares MP, Nilsson J, Pachinger O, Weidinger F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2003; 23:58–63. [DOI] [PubMed] [Google Scholar]
- 23. Buhaescu I, Izzedine H. Mevalonate pathway: a review of clinical and therapeutical implications. Clin Biochem 2007; 40:575–584. [DOI] [PubMed] [Google Scholar]
- 24. Piotrowski PC, Kwintkiewicz J, Rzepczynska IJ, Seval Y, Cakmak H, Arici A, Duleba AJ. Statins inhibit growth of human endometrial stromal cells independently of cholesterol availability. Biol Reprod 2006; 75:107–111. [DOI] [PubMed] [Google Scholar]
- 25. Esfandiari N, Khazaei M, Ai J, Bielecki R, Gotlieb L, Ryan E, Casper RF. Effect of a statin on an in vitro model of endometriosis. Fertil Steril 2007; 87:257–262. [DOI] [PubMed] [Google Scholar]
- 26. Sharma I, Dhawan V, Mahajan N, Saha SC, Dhaliwal LK. In vitro effects of atorvastatin on lipopolysaccharide-induced gene expression in endometriotic stromal cells. Fertil Steril 2010; 94:1639–1646.e1631. [DOI] [PubMed] [Google Scholar]
- 27. Nasu K, Yuge A, Tsuno A, Narahara H. Simvastatin inhibits the proliferation and the contractility of human endometriotic stromal cells: a promising agent for the treatment of endometriosis. Fertil Steril 2009; 92:2097–2099. [DOI] [PubMed] [Google Scholar]
- 28. Sokalska A, Wong DH, Cress A, Piotrowski PC, Rzepczynska I, Villanueva J, Duleba AJ. Simvastatin induces apoptosis and alters cytoskeleton in endometrial stromal cells. J Clin Endocrinol Metab 2010; 95:3453–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sokalska A, Cress A, Bruner-Tran KL, Osteen KG, Taylor HS, Ortega I, Duleba AJ. Simvastatin decreases invasiveness of human endometrial stromal cells. Biol Reprod 2012; 87:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oktem M, Esinler I, Eroglu D, Haberal N, Bayraktar N, Zeyneloglu HB. High-dose atorvastatin causes regression of endometriotic implants: a rat model. Hum Reprod 2007; 22:1474–1480. [DOI] [PubMed] [Google Scholar]
- 31. Bruner-Tran KL, Osteen KG, Duleba AJ. Simvastatin protects against the development of endometriosis in a nude mouse model. J Clin Endocrinol Metab 2009; 94:2489–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yilmaz B, Ozat M, Kilic S, Gungor T, Aksoy Y, Lordlar N, Sut N, Aksakal O. Atorvastatin causes regression of endometriotic implants in a rat model. Reprod Biomed Online 2010; 20:291–299. [DOI] [PubMed] [Google Scholar]
- 33. Cakmak H, Basar M, Seval-Celik Y, Osteen KG, Duleba AJ, Taylor HS, Lockwood CJ, Arici A. Statins inhibit monocyte chemotactic protein 1 expression in endometriosis. Reprod Sci 2012; 19:572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Rosa S, Cirillo P, Pacileo M, Petrillo G, D'Ascoli GL, Maresca F, Ziviello F, Chiariello M. Neopterin: from forgotten biomarker to leading actor in cardiovascular pathophysiology. Curr Vasc Pharmacol 2011; 9:188–199. [DOI] [PubMed] [Google Scholar]
- 35. Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab 2002; 3:175–187. [DOI] [PubMed] [Google Scholar]
- 36. Fuchs D, Avanzas P, Arroyo-Espliguero R, Jenny M, Consuegra-Sanchez L, Kaski JC. The role of neopterin in atherogenesis and cardiovascular risk assessment. Curr Med Chem 2009; 16:4644–4653. [DOI] [PubMed] [Google Scholar]
- 37. D'Hooghe TM, Nugent NP, Cuneo S, Chai DC, Deer F, Debrock S, Kyama CM, Mihalyi A, Mwenda JM. Recombinant human TNFRSF1A (r-hTBP1) inhibits the development of endometriosis in baboons: a prospective, randomized, placebo- and drug-controlled study. Biol Reprod 2006; 74:131–136. [DOI] [PubMed] [Google Scholar]
- 38. Hussein M, Chai DC, Kyama CM, Mwenda JM, Palmer SS, Gotteland JP, D'Hooghe TM. c-Jun NH2-terminal kinase inhibitor bentamapimod reduces induced endometriosis in baboons: an assessor-blind placebo-controlled randomized study. Fertil Steril 2016; 105:815–824.e815. [DOI] [PubMed] [Google Scholar]
- 39. D'Hooghe TM, Bambra CS, Raeymaekers BM, De Jonge I, Lauweryns JM, Koninckx PR. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons (Papio cynocephalus and Papio anubis). Am J Obstet Gynecol 1995; 173:125–134. [DOI] [PubMed] [Google Scholar]
- 40. D'Hooghe TM, Bambra CS, Cornillie FJ, Isahakia M, Koninckx PR. Prevalence and laparoscopic appearance of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus). Biol Reprod 1991; 45:411–416. [DOI] [PubMed] [Google Scholar]
- 41. Stratton P, Winkel CA, Sinaii N, Merino MJ, Zimmer C, Nieman LK. Location, color, size, depth, and volume may predict endometriosis in lesions resected at surgery. Fertil Steril 2002; 78:743–749. [DOI] [PubMed] [Google Scholar]
- 42. Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol 2006; 4(Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med 2010; 28:75–80. [DOI] [PubMed] [Google Scholar]
- 44. Harirchian P, Gashaw I, Lipskind ST, Braundmeier AG, Hastings JM, Olson MR, Fazleabas AT. Lesion kinetics in a non-human primate model of endometriosis. Hum Reprod 2012; 27:2341–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kurki P, Vanderlaan M, Dolbeare F, Gray J, Tan EM. Expression of proliferating cell nuclear antigen (PCNA)/cyclin during the cell cycle. Exp Cell Res 1986; 166:209–219. [DOI] [PubMed] [Google Scholar]
- 46. Yoshimura A, Inui K, Nemoto T, Uda S, Sugenoya Y, Watanabe S, Yokota N, Taira T, Iwasaki S, Ideura T. Simvastatin suppresses glomerular cell proliferation and macrophage infiltration in rats with mesangial proliferative nephritis. J Am Soc Nephrol 1998; 9:2027–2039. [DOI] [PubMed] [Google Scholar]
- 47. Koyuturk M, Ersoz M, Altiok N. Simvastatin induces proliferation inhibition and apoptosis in C6 glioma cells via c-jun N-terminal kinase. Neurosci Lett 2004; 370:212–217. [DOI] [PubMed] [Google Scholar]
- 48. Gao C, Xu W, Xiao W, Yu J, Li M. Simvastatin decreases stent-induced in-stent restenosis rate via downregulating the expression of PCNA and upregulating that of p27kip1. J Interv Cardiol 2013; 26:384–391. [DOI] [PubMed] [Google Scholar]
- 49. Chuang SC, Liao HJ, Li CJ, Wang GJ, Chang JK, Ho ML. Simvastatin enhances human osteoblast proliferation involved in mitochondrial energy generation. Eur J Pharmacol 2013; 714:74–82. [DOI] [PubMed] [Google Scholar]
- 50. Xia S, Kang J, Jiang Y, Huang D, Wang S, Pang B. Simvastatin promotes alveolar epithelial cell proliferation and attenuates cigarette smokeinduced emphysema in rats. Mol Med Rep 2015; 12:5903–5910. [DOI] [PubMed] [Google Scholar]
- 51. Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod 2007; 77:681–687. [DOI] [PubMed] [Google Scholar]
- 52. Sikora J, Mielczarek-Palacz A, Kondera-Anasz Z, Strzelczyk J. Peripheral blood proinflammatory response in women during menstrual cycle and endometriosis. Cytokine 2015; 76:117–122. [DOI] [PubMed] [Google Scholar]
- 53. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008; 22:659–661. [DOI] [PubMed] [Google Scholar]
- 54. Backes JM, Howard PA, Ruisinger JF, Moriarty PM. Does simvastatin cause more myotoxicity compared with other statins? Ann Pharmacother 2009; 43:2012–2020. [DOI] [PubMed] [Google Scholar]
- 55. Almassinokiani F, Mehdizadeh A, Sariri E, Rezaei M, Almasi A, Akbari H, Pazooki A, Solaymani-Dodaran M, Asadollah S, Amirkhani J, Chaichian S, Vahdat M et al. . Effects of simvastatin in prevention of pain recurrences after surgery for endometriosis. Med Sci Monit 2013; 19:534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Edison RJ, Muenke M. Mechanistic and epidemiologic considerations in the evaluation of adverse birth outcomes following gestational exposure to statins. Am J Med Genet A 2004; 131:287–298. [DOI] [PubMed] [Google Scholar]
- 57. Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Effects of simvastatin and metformin on polycystic ovary syndrome after six months of treatment. J Clin Endocrinol Metab 2011; 96:3493–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]


