Abstract
Purpose:
The purpose of this case series is to show the varied oral presentations of multiple myeloma, illustrating the importance of carefully surveying the oral cavity for suspicious lesions that could be indicative of palpable disease and/or recurrence. The diagnostic criteria and prognostic features for multiple myeloma were also reviewed.
Case Series Summary:
This report focuses on 5 patients with myeloma manifestations involving the oral cavity, in which the oral presentation of multiple myeloma was an early indication of disease relapse. Although the clinical presentation may be variable, the majority of patients will develop lytic bone lesions and less commonly, extramedullary involvement during the course of their disease.
Discussion:
The presentation of myeloma can be varied and the oral presentation, although rare, may be the sole manifestation, or part of a group of signs of disease progression. Clinical presentations of patients with myelomatous lesions can mimic common dental pathologies, which then, in turn, can lead to delays in diagnosis and treatment.
Conclusion:
As members of an interdisciplinary oncology team, it is essential to be familiar with oral manifestations of multiple myeloma and proper diagnostic/biopsy techniques in order to avoid misdiagnosis and treatment delays.
Keywords: Oral manifestations, multiple myeloma, extramedullary involvement, oral screening
Introduction
Multiple myeloma (MM) is characterized by a proliferation of malignant plasma cells and usually by production of a monoclonal immunoglobulin [1]. Nearly 20,000 new cases of this disease are diagnosed each year, representing approximately 1% of all cancers diagnosed annually [2]. Myeloma accounts for 10% of all hematologic malignancies and, if metastatic disease is excluded, approximately 50% of all malignancies involving bone [2] This disorder has a male predominance, and is twice as common in Blacks as in Whites. The median age at diagnosis is 65, but in many series, specialized referral centers may have a younger population of patients with multiple myeloma [3]. Although many etiologic factors have been described, including infectious agents like human herpes virus 8 (HSV-8), exposure to chronic low-dose ionizing radiation, and industrial exposure, case-control studies have failed to demonstrate a clear etiology for MM [1].
Myeloma is thought to be the result of a plasma cell that undergoes malignant transformation and proliferation that usually results in the production of a non-functional immunoglobin. This paraprotein may be found intact circulating in the serum, as free light chains in the serum and/or urine (Bence Jones protein), often resulting in an elevated total serum protein level or unexplained proteinuria [1]. Malignant plasma cells may interact with the marrow stroma and an extensive cytokine network resulting in osteolytic destruction of bone, or in some cases, producing extramedullary expansion or soft tissue plasmacytomas. In the absence of additional lesions, and other features of a systemic plasma cell disorder, fewer than 5% of patients with a plasma cell dyscrasia will be diagnosed with a solitary bone or extramedullary plasmacytoma [4,5] It is important to distinguish these patients from counterparts with myeloma since approximately 35–65% of these patients may be curable with definitive radiotherapy [5]
The majority of patients with a plasma cell dyscrasia have systemic disease (myeloma) diagnosed by International Myeloma Working Group criteria [6]. Diagnostic criteria for symptomatic MM include the presence of > 10% plasmacytosis in the bone marrow or biopsy-proven plasmacytoma; and evidence of end organ damage related to the underlying plasma cell disorder, commonly referred to as “CRAB [hyperCalcemia: corrected calcium >2.75 mmol/L, Renal insufficiency attributable to myeloma, anemia (hemoglobin <10 g/dL), and Bone lesions (lytic lesions or osteoporosis with compression fractures] criteria [6].
It appears that myeloma evolves from an asymptomatic premalignant stage termed monoclonal gammopathy of undetermined significance (MGUS) defined by a monoclonal seum protein of < 3 g/dL and < 10% marrow plasmacytosis in the absence of myeloma-related organ or tissue impairment [6]. This disorder is present in over 3% of the population above the age of 50 and progresses to myeloma or a related malignancy at a rate of approximately 1–2% per year [6].
Most patients with a plasma cell dyscrasia present with myeloma manifest by a variety of symptoms including anemia (+/− fatigue) usually due to marrow infiltration, renal insufficiency/failure, recurrent infections secondary to reduced uninvolved immunoglobulins, lytic bone lesions, and/or hypercalcemia. Seventy percent of patients present with lytic bone disease visible radiographically at diagnosis; findings can include well-defined punched-out lesions or less demarcated radiolucent lesions [6]. The diagnosis is confirmed by demonstrating >10% marrow plasmacytosis and a monoclonal immunoglobulin on serum or urine immunofixation studies, which can be further quantified by serum or urine protein electrophoresis [1]. Less commonly the disease may be manifest orally with jaw or tooth pain, paresthesias, swelling, soft-tissue masses, mobility of teeth, migration of teeth, hemorrhage, and pathologic fracture due to cortical destruction of bone [7].
The International Staging System for myeloma based on beta 2 -microglobulin and albumin was introduced in 2005 by Greipp et al, and is currently considered the standard for initial staging of patients with myeloma [8]. This system defines 3 stages of disease with varied prognosis. Stage I disease is characterized by a β−2 microglobulin < 3.5 mg/L and albumin ≥ 3.5 g/dL, stage 2 by a β−2 microglobulin ≥ 3.5 mg/L, but < 5.5 mg/dL and/or albumin ≤ 3.5 g/dL, and stage 3 by a β−2 microglobulin ≥5..5 mg/L. Cytogenetic abnormalities such as gains or losses of chromosome 1, deletion of chromosome 13, translocation of chromosome 14 with either chromosome 4, 16, or 20, and deletion of chromosome 17p provide additional prognostic information for increased resistance and shorter survival among myeloma patients [9].
Multiple myeloma is generally considered incurable and is usually characterized by frequent relapses requiring therapy. Induction treatment usually consists of doublets or triplets of steroids, immunomodulatory agents, with or without alkylating agents or anthracyclines; the most common regimens include dexamethasone and one or more novel agents like bortezomib (Millennium Pharmaceuticals, Cambridge, MA), carfilzomib, lenalidomide (Celgene, Summit NJ), pomalidomide, or thalidomide with or without cyclophosphamide and/or anthracyclines (or liposomal anthracyclines for induction followed by consolidation with myeloablative therapy and autologous stem cell transplant (ASCT)). Melphalan (Celgene, Summit NJ) may be used for induction in patients not eligible for stem cell transplant, but because of its myelosuppressive effects (particularly on stem cells), is generally avoided until after stem cell harvest for transplant candidates, for whom it is usually used later as part of myeloablative therapy. With improvements in overall response rates to 90–100% after induction therapy with novel regimens, it is now possible to reserve radiation therapy for use in patients with cord compression or impending fracture in an effort to preserve collectability of stem cells.
Patients with symptomatic myeloma who require treatment for their disease are also treated with bisphosphonates to reduce skeletal related events such as pain or fractures [10]. Recommendations for the length and frequency of therapy, with or without maintenance vary. Previous treatment with these agents were reserved for patients with bone lesions, but recently a large randomized trial demonstrated a modest benefit in median survival for all patients receiving bisphosphonates, arguably justifying their use in most patients with myeloma [11]. Additionally, these agents may be used intermittently to treat hypercalcemia associated with myeloma. There are oral complications associated with these therapies such as immunosuppression associated higher rates of caries and periodontal disease and an increased incidence of bisphosphonate-related osteonecrosis of the jaw.
The development of bisphosphonate-related osteonecrosis is low, approximately 4%; however, this challenge is multi-factorial in etiology with varied clinical presentations and must be distinguished from pathology of the oral cavity due to myeloma [12]. Careful surveillance of the oral cavity has been recommended previously in the literature for suspicious lesions that could be indicative of palpable disease and/or recurrence [13–15]. The challenge with such patients is that oral manifestations of myeloma can mimic those of common oral/dental infection that can subsequently lead to delays in therapy. The purpose of this case series is to present the varied oral presentations of relapsed MM.
CASE REPORTS
Case #1 Painless Swelling of the Maxilla
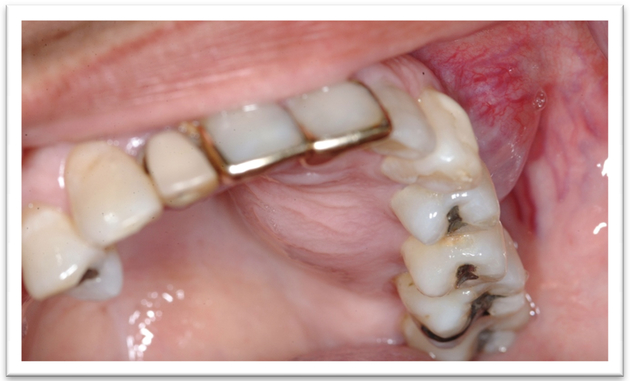
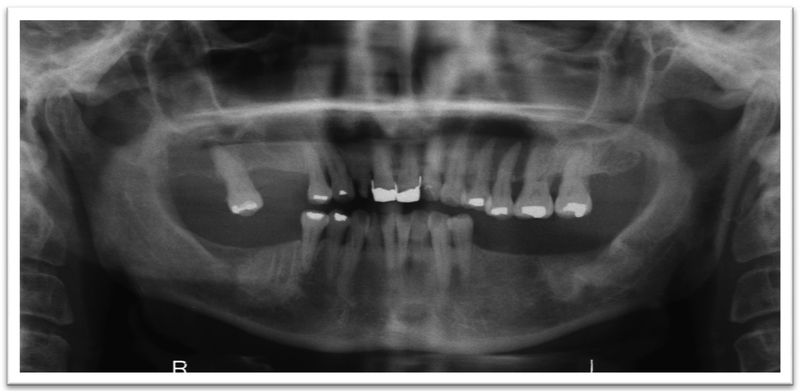
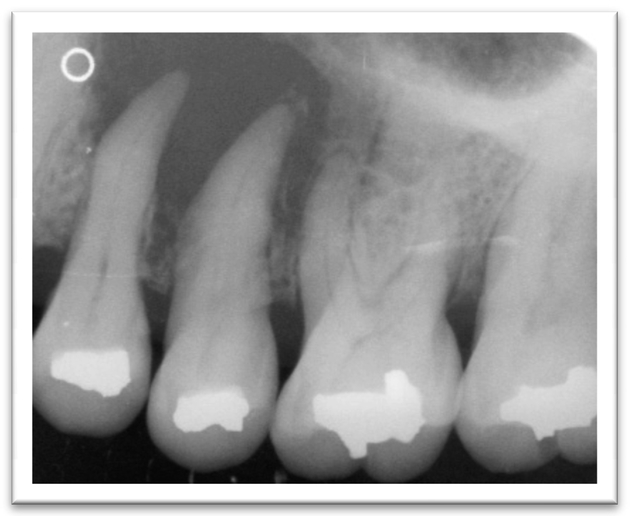
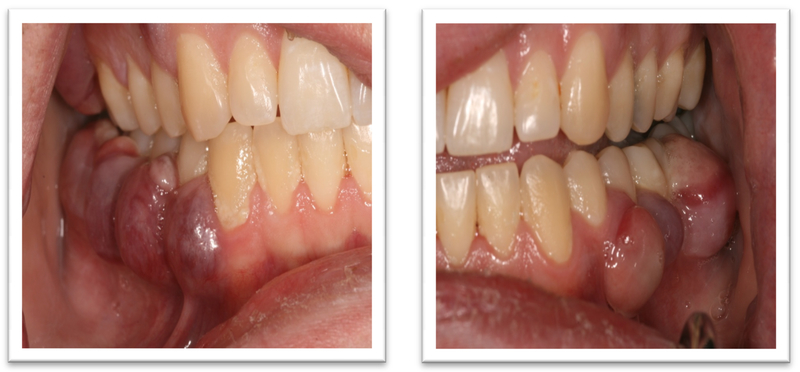
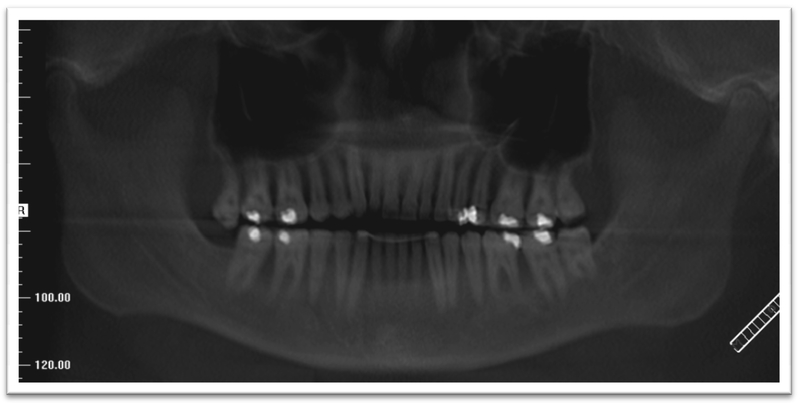
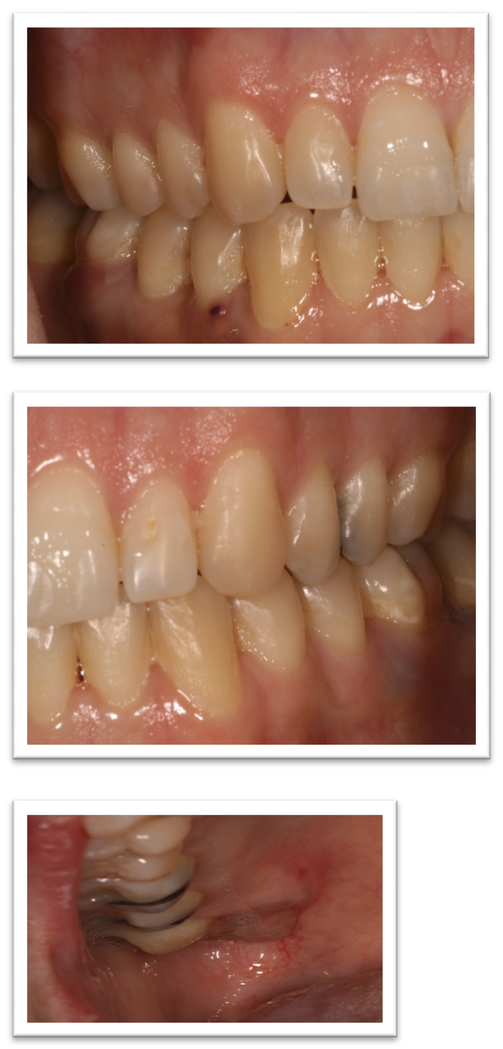
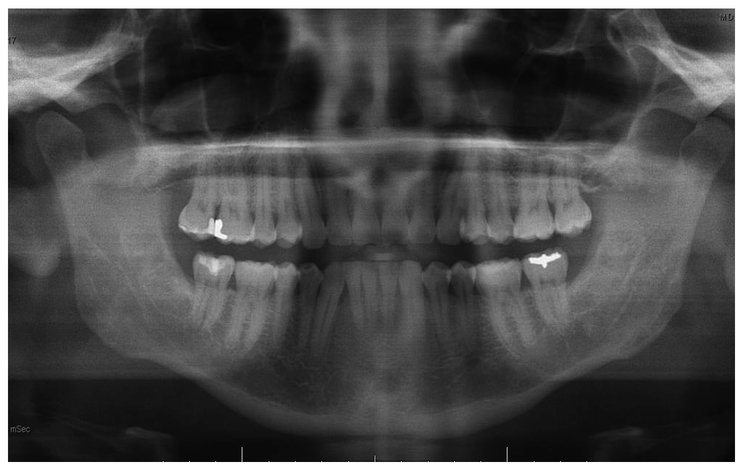
A 52-year old woman presented to the Oral Oncology Clinic at MD Anderson Cancer Center with a chief complaint of numbness in her right lower lip and chin that had begun approximately 5 days previously. She had a painless swelling in her left maxilla that she attributed to trauma from eating. Intra-oral examination revealed a large mass of the left maxilla that involved both the premolars and the first molar extending buccally and palatally (Fig. 1). Although she denied any dentition-related symptoms, on examination teeth #12 and #13 (universal numbering system) had class II mobility. A panoramic radiograph showed an ill-defined radiolucency that extended superiorly towards the maxillary sinus involving the roots of teeth #12 and #13 (Fig. 2). A periapical radiograph showed the radiolucency to be ill defined. There appeared to be no lamina dura around the first premolar and the lamina dura around the second premolar appeared mottled (Fig. 3). An FNA of the maxillary lesion was performed and revealed plasma cell infiltration.
Figure 1.
Initial presentation of patient #1.
Figure 2.
Initial panoramic radiograph of the maxilla and mandible.
Figure 3.
Periapical radiograph revealing the maxillary radiolucency.
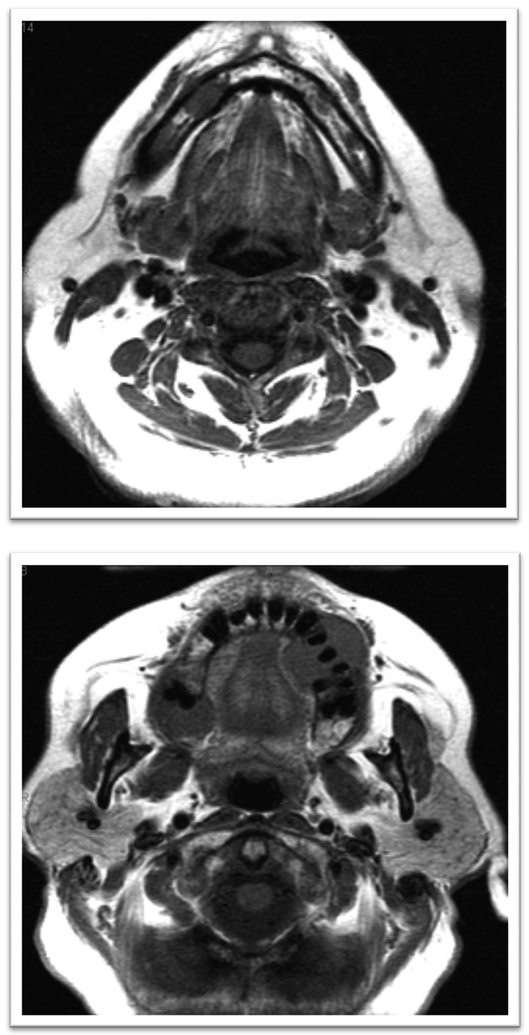
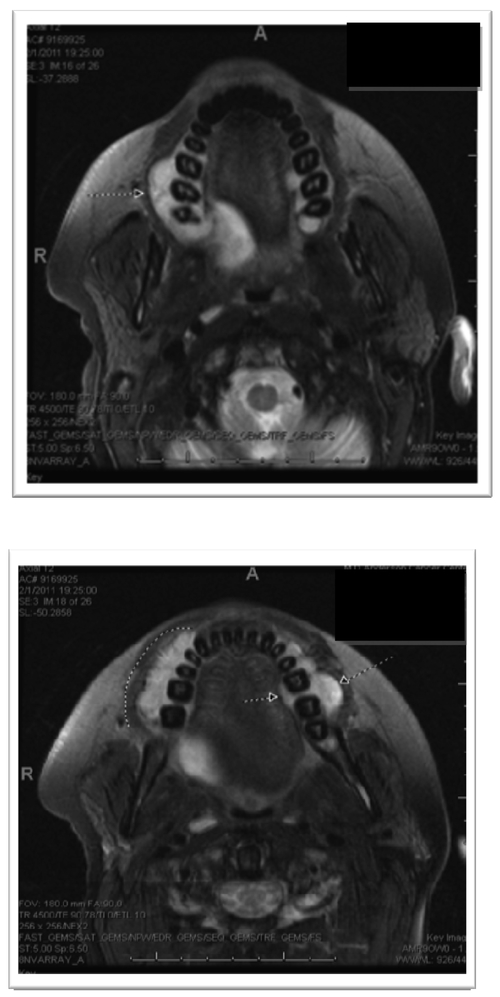
Magnetic resonance imaging (MRI) was performed and demonstrated several maxillary lesions including a 1.4-cm lesion on the right anterior region, a 2-cm lesion within the right posterior maxilla, and a 3-cm lesion on the left; also several lesions were noted on the mandible that were not clinically visible (Figs. 4a, 4b). External-beam radiation therapy was prescribed with lateral appositional field coverage. No chemotherapy was prescribed at that time. At the patient’s request, due to mucositis and dysphagia, radiation therapy was discontinued after 20-Gray (Gy) of radiation in 8 fractions using 6-MV photons; however, this limited therapy resulted in resolution of her neuropathy and maxillary swelling. An MRI scan was repeated after radiation therapy and revealed resolution of the lesion within the left maxillary alveolus with stability of the remaining lesions (Figs. 5a, 5b). The patient was subsequently lost to follow-up.
Figures 4a, 4b.
Magnetic resonance imaging without contrast (pre-treatment) revealing maxillary enhancement.
Figures 5a, 5b.
MRI without contrast post treatment.
Case #2 Painless Gingival Masses
A 31-year-old man with previously treated non-oral solitary plasmacytomas presented to the Oral Oncology Clinic for evaluation of gingival masses. At his initial presentation in the summer of 2010, he complained of back pain, was subsequently evaluated by MRI, which revealed a spinal tumor; subsequently, after laminectomy, pathology confirmed malignant plasmacytosis of the solitary lesion and laboratory evaluation confirmed an IgG paraprotein. The patient was treated with external beam radiotherapy of curative intent for solitary plasmacytoma [40 Gy in 25 fractions]. Subsequently, he developed an acneiform lesion above the left eye, that was biopsied and a plasmacytoma was confirmed. This was treated with involved field radiation therapy (total dose 30 Gy). Bone marrow biopsy revealed plasmacytosis, confirming systemic disease, and therapy with lenalidomide and dexamethasone followed by vincristine, doxorubicin and dexamethasone was prescribed. After 3 cycles of therapy, disease progression was confirmed after the patient complained of diplopia and a 6th nerve palsy was evident on clinical exam and confirmed with a lesion detected by MRI of the brain; the patient was then treated with palliative external beam radiotherapy.
The MRI specifically revealed presumed myelomatous involvement in the left central skull base with extension to the left middle cranial fossa, anterior displacement of the left Meckel’s cave with probable posterior invasion, involvement in the left internal auditory canal, and an enhancing focus in the left parietal calvarium; bone survey showed radiographic evidence of myelomatous involvement in the calvarium, ribs, pelvis, and probable left proximal femur. Lumbar puncture did not reveal malignant plasma cells, but atypical lymphocytes were detected.
A bone marrow biopsy confirmed progressive plasmacytosis. An Immunoglobulin G (IgG) kappa M-protein (paraprotein) of 0.5 with an elevated free kappa-to lambda ratio (immunoglobulin light chain) of 105.6 was detected without the presence of Bence Jones proteinuria. Treatment with bortezomib, cyclophosphamide, and dexamethasone was initiated and his visual symptoms improved, but did not completely correct. An MRI of the skull base revealed improvement in left skull base infiltration reflecting a partial response, but the patient had persistent diplopia, which was corrected by prism glasses. After 3 courses of chemotherapy, the patient underwent consolidative myeloablative therapy using high-dose melphalan and autologous stem cell transplant in early December 2010. Approximately 6 weeks post-therapy, he developed pain in multiple areas, difficulty swallowing, and several oral lesions.
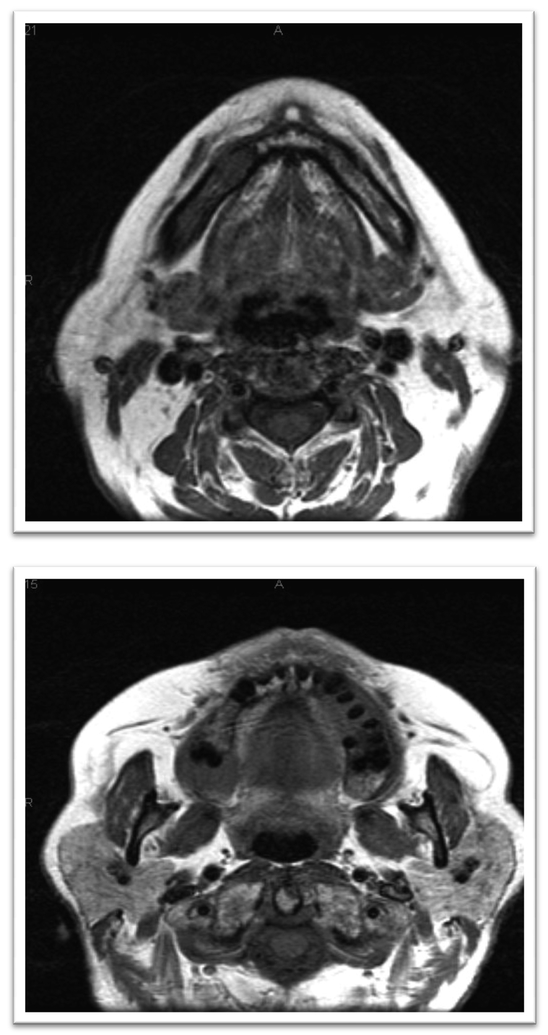
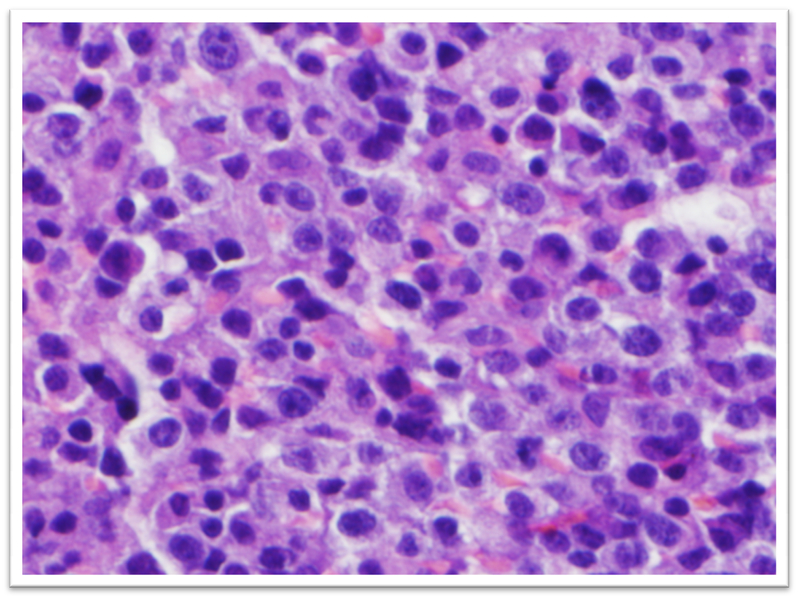
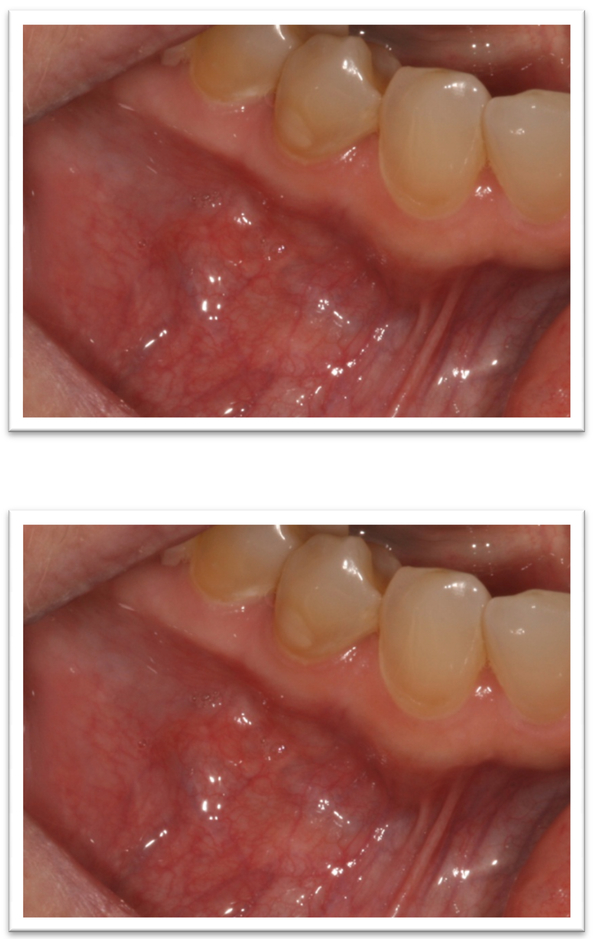
The patient was referred to Oral Oncology Clinic with oral pathology (Fig. 6a, 6b). Cone-beam computerized tomography (CBCT) was performed and revealed two localized ill-defined radiolucent lesions associated with tooth #19 and #30 (Fig 7). Intra-orally he presented with several gingival masses associated with most of his posterior dentition. He had a large mass on the soft palate nearly obstructing the airway. After hematologic evaluation, it was determined that it was safe for this patient to undergo excisional biopsy. Biopsy of the lesion revealed a dense infiltrate of plasma cells with enlarged eccentric nuclei, prominent nucleoli, and moderate cytoplasm (Fig. 8). An MRI (Fig. 9) of the head and neck showed extensive myelomatous involvement in the right maxilla with involvement of the right posterior maxillary and buccal gingiva that correlated clinically with the exophytic mass. The patient received a total of 3 courses of bortezomib with DT-PACE (dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, etoposide). After one cycle, he had partial resolution of his intra-oral lesions (Fig. 10a–c). However, CT of the abdomen and pelvis showed progression of other soft tissues including progressive retroperitoneal lymphadenopathy, splenomegaly, and lytic bone lesions. The patient continued active therapy, but developed obtundation after subdural hematoma and a decision was made for palliative care only considering his progressive myeloma and he subsequently expired 5 months after the oral lesions were noted.
Figure 6a, 6b.
Initial presentation of case #2.
Figure 7.
Initial radiograph showing some localized bone loss associated with #19 and 30.
Figure 8.
Dense infiltrate composed of plasma cells with enlarged eccentric nuclei, prominent nucleoli, and moderate of cytoplasm
Figure 9a, b.
MRI revealing several buccal maxillary and mandibular lesions; also, a large pharyngeal lesion
Figure 10a, 10b, 10c.
Partial resolution of intra-oral lesions.
Case #3 Evaluation of Mandibular Numbness
A 70-year-old female with a history of IgG lambda myeloma with normal cytogenetics was referred to the Oral Oncology Clinic for evaluation of mandibular numbness. The patient had previously been treated with multiple regimens including bortezomib, lenalidomide and dexamethasone, lenalidomide maintenance, hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone (hyperCVAD) and myeloablative therapy with autologous stem cell transplant. The patient developed an exophytic mass on her right mandible at the location of the mental foramen.
Her clinical history included several months of paresthesia in the trigeminal distribution of division III that did not cross the midline in the right lower face. Upon examination, the patient was found to have a palpable and freely mobile mass in the right gingivobuccal sulcus between the second and first premolars (Fig. 11). The patient had mild tenderness upon palpation.
Figure 11.
Initial presentation.
An excisional biopsy was performed of the oral lesion and pathology revealed fibroadipose tissue with diffuse infiltration by malignant neoplasm. The neoplasm had a diffuse pattern and was composed of large cells with eccentric, round vesicular nuclei, prominent nucleoli, with abundant cytoplasm consistent with plasma cells. Despite continued chemotherapy the patient died of progressive myeloma 3 months later.
Case #4 Evaluation of Maxillary Mass
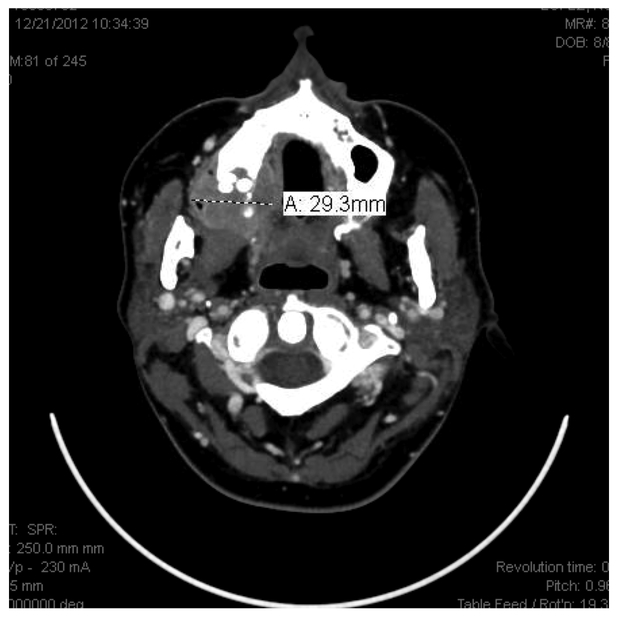
A 42 year old presented to the Oral Oncology Service initially for clearance for bisphosphonates in 04/2011, now with mass in the right maxilla. She initially presented to MDACC in 03/2011 with persistent diplopia, photophobia, and headaches. Laboratory examination confirmed hypercalcemia as well as Bence Jones proteinuria and an IgG lambda multiple myeloma with high-risk cytogenetics including deletion of chromosome 13, deletion of chromosome 17p and t (4; 14). Subsequently, MRI of the brain revealed extensive calvarial metastatic disease with areas of extraosseous soft tissues component. A CT of the head and neck revealed an enhancing mass involving the left sphenoid bone with extension into the middle cranial fossa and the posterior left orbit. She was initially treated with palliative radiation therapy of 24Gy in 12 daily fractions in combination with bortezomib and dexamethasone. Lenalidomide was added upon completion of radiotherapy.
A bone survey demonstrated multiple lesions on the humeri and proximal radius, and the patient began bisphosphonate therapy after clearance from the 0ral Oncology Service. The patient achieved a very good partial remission and underwent consolidative therapy with myeloablative therapy and tandem autologous stem cell transplantation. Within one month she was found to have progressive disease and bortezomib, lenalidomide, and dexamethasone was reinitiated for relapsing disease.
In 05/12 she later developed a 1.5cm lesion the right maxilla; a biopsy revealed sheets of plasma cells of small to medium size with eccentrically located nuclei, dense nuclear chromatin and an abundant cytoplasm with perinuclear hof. She was subsequently treated with modified hyper-CVAD. While the oral maxillary mass showed resolution, she was found to have progressive disease with a new lesion on the right mandible resulting in jaw pain extending into the orbit and lip anesthesia. She was then treated with Dexamethasone, Thalidomide, cisplatin, cyclophosphamide, doxorubicin and etoposide, but developed progressive disease. She continued therapy with lenalidomide, thalidomide and dexamethasone as well as palliative radiation therapy for the mandibular lesion. The patient subsequently developed progressive complications and elected to proceed with supportive care only due to progressive disease. She expired approximately 8 months after the discovery of oral lesions.
Case #5 Evaluation of Bilateral Mandibular Mass
A 55-year-old male presented to MDACC for a second opinionregarding an IgA lambda multiple myeloma with normal cytogenetics initially treated with lenalidomide and dexamethasone. He had rapidly progressive bone disease in the left sacrum treated with radiation therapy.
Subsequently he was found to have bilateral swelling of the mandible. Radiographically there was slight, multilocular radiolucency in the areas of the masses. Salvage chemotherapy was initiated with hyper-CVAD followed by remission; he subsequently continues in remission 2 months later with planned consolidative meyloablative therapy followed by autologous stem cell support.
Discussion
As members of the interdisciplinary oncology team, it is essential to carefully survey the oral cavity for suspicious lesions that could be indicative of palpable disease and/or recurrence. There is a wide range in the reported incidence of oral manifestations of myeloma. A review of the literature has shown that approximately 14% of patients will have oral manifestations; this is felt to be a low estimate since most records were medical without dental consult [7]. When patients present with an oral lesion and a history of myeloma, an oral manifestation of the disease must be part of the differential diagnosis.
The presentation of myeloma can be varied and the oral presentation, although rare, may be the sole manifestation, or part of a group of signs of disease progression. Clinical presentations of patients with myelomatous lesions can mimic common dental pathologies, i.e., Case #1 could be misdiagnosed as a peri-apical or periodontal abscess and Case #2 misdiagnosed as severe gingivitis or periodontitis. The clinical appearance of each of the lesions were different in that case #1 had what appeared to be normal mucosa both in color and texture while in case #2 the gingiva was distinctly different, presenting as multiple small lesions, each smooth and purple in color. In contrast, case #3 was difficult to detect, both clinically and radiographically. The traditional punched out, osteolytic lesion was difficult to confirm on imaging, both panoramic radiograph and MRI. Among the varied presentations in this series, the common thread among all the cases was anesthesia or paraesthesia of the lips and affected areas. It would be unlikely that a periodontal or peri-apical abscess would cause alterations in sensation; however, these should never be excluded from the differential diagnoses.
While excisional/incisional biopsy remains the gold standard for diagnosis of myelomatous lesions, there is evidence to suggest that a FNA biopsy is a viable option in the early diagnosis of a suspicious mass/lesion. The advantages of an FNA biopsy are ease of execution, low complication rate, and rapid diagnosis [16–17]. One benefit of the FNA biopsy is it can provide a specimen for analysis in situation where an excisional/incisional biopsy could pose significant morbidity or hematologic challenge.
In this limited series, there was a slight predominance of lambda light chain (3 of 4 patients with immunofixation) and IgG clonality (3 of 4 patients with immunofixation). Although, overall survival after discovery of oral manifestations of myeloma in this small series of patients was short, only one patient with evaluable cytogenetic data, had evidence of high-risk disease. Although further studies of larger series of patients are necessary to confirm the poor prognosis of these patients, the short survival of patients in this small series, despite therapy with immunomodulatory agents and proteasome inhibitors, seems to justify an aggressive approach with novel therapeutics for patients with oral plasmacytomas.
Conclusion
Lesions in the oral cavity can be the first sign of recurrence or progression of multiple myeloma and thus, members of the interdisciplinary oncology team should be intimately aware of the various signs, symptoms and treatment MM and its various presentations within the oral cavity. Prompt recognition of oral manifestations of disease, followed by effective coordination of the multidisciplinary team is essential for patients care. Although, this represents a small series of patients, the relatively short survival after discovery of an oral lesion, appears to justify further study to determine the most effective therapies for patients with this manifestation of myeloma.
Figure 12.
Initial radiographic presentation.
Figure 13.
Clinical Initial Presentation
Footnotes
Conflicts of interest
None of the authors has any financial and personal relationships with other people or organizations that could inappropriately influence their work.
Contributor Information
Richard C. Cardoso, Dept. of Head and Neck Surgery; Section of Oral Oncology, The University of Texas—M.D. Anderson Cancer Center.
Peter J. Gerngross, Maxillofacial Prosthodontics Dental Service, Michael E. DeBakey—VA Medical Center.
Theresa M. Hofstede, Dept. of Head and Neck Surgery; Section of Oral Oncology The University of Texas—M.D. Anderson Cancer Center.
Donna M. Weber, Department of Lymphoma/Myeloma The University of Texas—M.D. Anderson Cancer Center.
Mark S. Chambers, Dept. of Head and Neck Surgery; Section of Oral Oncology The University of Texas—M.D. Anderson Cancer Center.
References
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE (2009) Hematologic Disorders In: Oral and Maxillofacial Pathology. 3rd ed., W.B. Saunders-Elsevier, Philadelphia: pp 526–8. [Google Scholar]
- 2.Rajkumar SV (2011) Multiple myeloma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol 86: 57–65. doi: 10.1002/ajh.21913 [DOI] [PubMed] [Google Scholar]
- 3.Nofsinger YC, Mirza N, Rowan PT, Lanza D, Weinstein G (1997) Head and neck manifestations of plasma cell neoplasms. Laryngoscope 107:741–6 [DOI] [PubMed] [Google Scholar]
- 4.Weber DM (2005) Solitary Bone and extramedullary plasmacytoma. Hematology Am Soc Hematol Educ Program. doi: 10.1182/asheducation-2005.1.373 [DOI] [PubMed] [Google Scholar]
- 5.Reed V, Shah J, Medeiros LJ, Ha CS, Mazloom A, Weber DM, Arzu IY, Orlowski RZ, Thomas SK, Shihadeh F, Alexanian R, Dabaja BS (2011) Solitary plasmacytomas: outcome and prognostic factors after definitive radiation therapy. Cancer 117: 4468–74. doi: 10.1002/cncr.26031. [DOI] [PubMed] [Google Scholar]
- 6.International Myeloma Working Group (2003) Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a reports of the International Myeloma working group. Br J Haematol 121: 749–57 [PubMed] [Google Scholar]
- 7.Epstein JB, Voss NJS, Stevenson-Moore P (1984) Maxillofacial manifestations of multiple myeloma. An unusual case and review of the literature. Oral Surg Oral Med Oral Pathol 57: 267–271. [DOI] [PubMed] [Google Scholar]
- 8.Greipp PR, San Miguel J, Durie BG, Crowley JJ, et al. (2005) International staging system for multiple myeloma. J Clin Oncol 23: 3412–20 [DOI] [PubMed] [Google Scholar]
- 9.Herve AL, Florence M, Philippe M, Michel A, et al. (2011) Molecular Heterogeneity of multiple myeloma: pathogenesis, prognosis, and therapeutic implications. J Clin Oncol 29:1893–7. doi: 10.1200/JCO.2010.32.8435 [DOI] [PubMed] [Google Scholar]
- 10.Mhaskar R, Redzepovic J, Wheatley K, Clark OA, Miladinovic B, Glasmacher A, Kumar A, Djulbegovic B. (2010) Bisphosphonates in multiple myeloma (Review). Cochrane Database Syst Rev. 17 doi: 10.1002/14651858.CD003188 [DOI] [PubMed] [Google Scholar]
- 11.Morgan GJ, Davies FE, Gregory WM Cocks K et al. (2010) First-line treatment with zoledronic acid as compared with cldronic acid in multiple (MRC Myeloma IX): a randomised controlled trial. Lancet 376: 1989–99. doi: 10.1016/S0140-6736(10)62051-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto S, Schereyer C, Hafner S, Mast G, Ehrefeld M, Sturzenbaum S, Pautke C (2012) Bisphosphonate-related osteonecrosis of the jaws—Characteristics, risk factors, clinical features, localization and impact on oncological treatment. J Craniomaxillofacial Surg 40: 303–9. doi: 10.1016/j.jcms.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 13.Lambertenghi-Deliliers G, Bruno E, Cortelezzi A, Fumagalli L, Morosini A (1988) Incidence of jaw lesions in 193 patients with multiple myeloma. Oral Surg Oral Med Oral Pathol 65:533–537 [DOI] [PubMed] [Google Scholar]
- 14.Furutani M, Ohnishi M, Tanaka Y (1994) Mandibular involvement in patients with multiple myeloma. J Oral Maxillofac Surg 53:23–25 [DOI] [PubMed] [Google Scholar]
- 15.Pisano JJ, Coupland R, Chen SY, Miller AS (1997) Plasmacytoma of the oral cavity and jaws. A clinicopathologic study of 13 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 83:265–271 [DOI] [PubMed] [Google Scholar]
- 16.Daneshbod Y, Arabi MA, Ramzi M, Daneshbod K (2008) Jaw lesion as the first presentation of multiple myeloma diagnosed by fine needle aspiration. Acta Cytol 52:268–70 [DOI] [PubMed] [Google Scholar]
- 17.August M, Faquin WC, Ferraro NF, Kaban LB (1999) Fine-Needle Aspiration Biopsy of Intraosseous Jaw Lesion. J Oral Maxillofac Surg 57; 1282–6 [DOI] [PubMed] [Google Scholar]