Abstract
Exon-skipping antisense oligonucleotides are effective treatments for genetic diseases, yet exon-skipping activity requires that these macromolecules reach the nucleus. While cell-penetrating peptides can improve delivery, proteolytic instability often limits efficacy. We hypothesized that bicyclization of arginine-rich peptides would improve their stability and their ability to deliver oligonucleotides into the nucleus. Here, we introduce two methods for the synthesis of arginine-rich bicyclic peptides using cysteine perfluoroarylation chemistry. Then, the bicyclic peptides are covalently linked to a phosphorodiamidate morpholino oligonucleotide (PMO) and assayed for exon skipping activity. The perfluoroaryl cyclic and bicyclic peptides improve PMO activity roughly 14-fold over the unconjugated PMO. The bicyclic peptides exhibited increased proteolytic stability relative to the monocycle, demonstrating that perfluoroaryl bicyclic peptides are potent and stable delivery agents. Exon-skipping antisense oligonucleotides are effective treatments for genetic diseases, yet exon-skipping activity requires that these macromolecules reach the nucleus. While cell-penetrating peptides can improve delivery, proteolytic instability often limits efficacy. We hypothesized that bicyclization of arginine-rich peptides would improve their stability and their ability to deliver oligonucleotides into the nucleus. Here, we introduce two methods for the synthesis of arginine-rich bicyclic peptides using cysteine perfluoroarylation chemistry. Then, the bicyclic peptides are covalently linked to a phosphorodiamidate morpholino oligonucleotide (PMO) and assayed for exon skipping activity. The perfluoroaryl cyclic and bicyclic peptides improve PMO activity roughly 14-fold over the unconjugated PMO. The bicyclic peptides exhibited increased proteolytic stability relative to the monocycle, demonstrating that perfluoroaryl bicyclic peptides are potent and stable delivery agents.
Keywords: antisense agents, bicyclization, cell-penetrating peptides, cysteine arylation, peptides
Assembling a new bicycle:
Peptide bicycles are synthesized using cysteine perfluoroarylation chemistry. The bicyclic peptides demonstrate enhanced proteolytic stability relative to the monocyclic peptide. Additionally, after conjugation to antisense oligonucleotides, the bicyclic peptide conjugates exhibit a 14-fold increase in cellular activity when compared to the unconjugated oligonucleotide.
Graphical Abstract
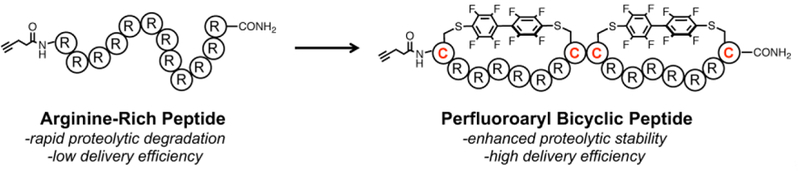
Exon-skipping antisense oligonucleotides have been developed into successful therapeutic molecules for debilitating genetic diseases, such as Duchenne muscular dystrophy (DMD).[1–3] To promote exon-skipping, these molecules bind to pre-mRNA in the nucleus and sterically block splice junctions or intraexonic ribonucleoprotein binding motifs. The steric blocking alters the spliceosomal processing of pre-mRNA, and results in the exclusion of one or more exons from the mature mRNA transcript. Thus, the oligonucleotide restores the in-frame mRNA and leads to translation of a functional protein.[1]
Phosphorodiamidate morpholino oligonucleotides (PMOs) are one type of exon-skipping oligonucleotide. PMOs are uncharged nucleic acid polymers in which the ribosyl ring is replaced with a morpholino ring and the natural phosphodiester backbone is replaced with phosphorodiamidate.[4] PMOs readily hybridize to DNA and RNA, and their altered backbone structure prevents degradation by serum and intracellular nucleases.[5,6] Eteplirsen, a PMO designed to restore functional dystrophin, recently became the only FDA-approved therapy to address the underlying genetic cause of DMD.
Unfortunately, PMOs, like all antisense oligonucleotides, exhibit limited delivery into the nucleus of cells. To improve PMO delivery, multiple strategies have been explored, such as the covalent attachment of cationic dendrimers or side-chains.[7–9] Arginine-rich cell-penetrating peptides have been widely utilized to improve cargo delivery, and these peptides are one of the most promising strategies for PMO delivery.[10–14] However, arginine-rich sequences suffer from proteolytic instability.[6] Delivery tools with enhanced proteolytic stability and delivery efficiency are therefore widely desired.
Bicyclic peptides are a unique class of peptides characterized by two intramolecular linkages. Excluding disulfide-linked peptides, methods to bicyclize unprotected peptides include bromomethylated aromatics, thiol-ene chemistry, tetrafluoroterephthalonitrile, or multiple orthogonal reactions.[15–19] The constrained conformation of bicyclic peptides can lead to reduced flexibility, improved binding properties, and protease resistance. Additionally, in the context of cellular delivery, bicyclic peptides with certain sequences show robust uptake into cells.[20,21] However, bicyclic peptides have not been explored as tools to promote the intracellular delivery of a large, covalently-attached cargo such as PMOs.
We recently developed a new method for peptide macrocyclization which joins the cysteine side-chains of unprotected peptides with perfluoroaromatic linkers.[22,23] These perfluoroaryl macrocyclic peptides have increased uptake into cells and increased proteolytic stability. Because the synthesis of multiple sizes of macrocycles is facile, we envisioned this perfluoroarylation chemistry could be extended towards the synthesis of bicyclic peptides. We anticipated that these bicyclic peptides would have enhanced stability, and that when covalently attached to oligonucleotides, would promote intracellular delivery.
Our first peptide bicyclization strategy used a trithiol to link together three perfluoroarenes covalently attached to an unprotected peptide (Figure 1). We began with R12 peptide 1. The purified peptide was incubated with an excess of decafluorobiphenyl in dimethylformamide (DMF) with N,N-diisopropylethylamine (DIEA) to generate the perfluoroarylated peptide 1a (35% isolated yield). After purification of 1a, 1,3,5-benzenetrithiol (1 mM) was added to the peptide (1 mM) in DMF and after 2 hours the bicyclic peptide 1b was observed. The reaction was quenched and the peptide was purified by high-performance liquid chromatography (HPLC) (62% isolated yield). This strategy also enables the bicyclization of smaller peptides, demonstrated by its applicability to R6 peptide 2. This peptide was reacted with excess decafluorobiphenyl and then bicyclized under similar conditions to generate peptide 2b (35% isolated yield) (SI Fig. 1).
Figure 1: Multiple cysteine residues coupled to a perfluoroarene can be linked with 1,3,5-benzenetrithiol to enable peptide bicyclization.
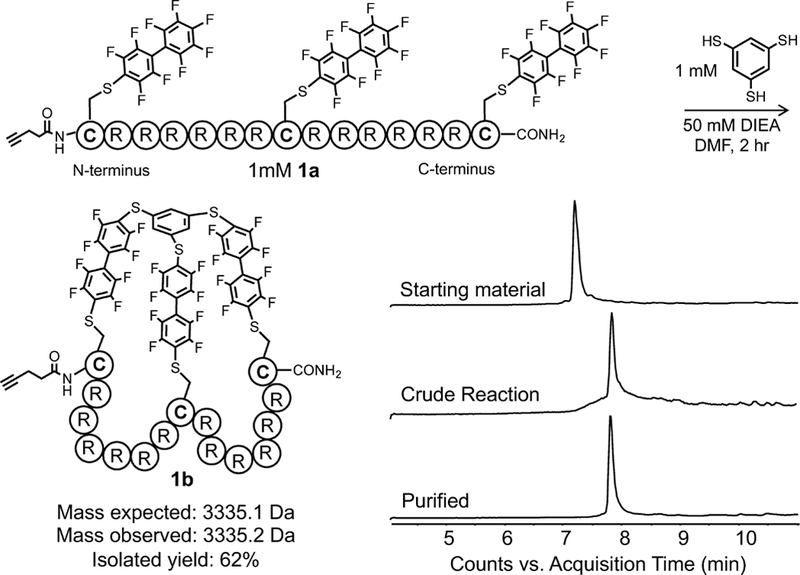
Peptide 1 is perfluoroarylated to give peptide 1a. Incubating 1a (1 mM) with equimolar 1,3,5-benzenetrithiol for 2 hours generates the bicyclic peptide 1b. LC-MS analysis confirms complete conversion. All chromatograms are total ion currents (TIC). HPLC purification affords 1b in 62% yield.
For a second bicyclization strategy, we performed a double macrocyclization of a linear peptide. Initially, we synthesized the R12 peptide 3 and utilized orthogonal protection to install two macrocycles sequentially (SI Fig. 2). However, after final purification, the bicyclic peptide 3b was obtained in an overall yield of 4.5%.
To improve the synthesis of 3b by eliminating deprotection and purification steps, we developed an alternative method, which we refer to as kinetically controlled bicyclization (Figure 2A). Given the slow rates of i, i+1 cyclization with decafluorobiphenyl,[23] we anticipated unlinked perfluoroarenes could be pre-installed on resin and peptide cyclization would be favored at specific sites after the addition of base. Peptide 3 was synthesized with two trityl-protected cysteine residues and two tert-butylthiol- protected cysteine residues. The tert-butylthiol protected cysteines were placed at positions 1 and 9 and deprotected on resin. Then, decafluorobiphenyl was introduced and allowed to react with the free thiol. Cleavage and HPLC purification provided peptide 3a. Next, the peptide was simply dissolved in DMF with 50 mM DIEA. Complete conversion to the double cycle product 3b occurred in under 5 minutes (Figure 2B). HPLC purification led to the isolation of 3b in 81% yield.
Figure 2: Kinetically controlled bicyclization enables high-yield synthesis of a double perfluoroaryl macrocyclic peptide.
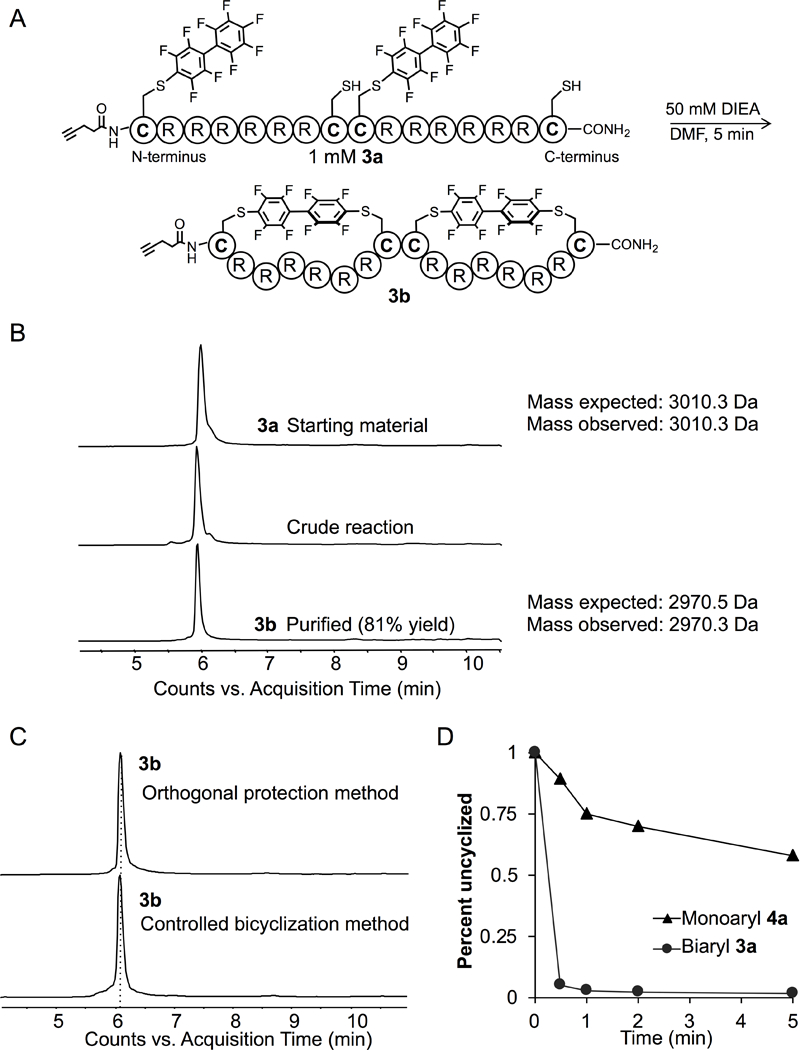
A) Two perfluoroarenes are selectively installed on the peptide on resin. Peptide 3a undergoes intramolecular SNAr after addition of base to preferentially form two i, i+7 cycles, due to the slow rate of linking adjacent i, i+1 residues. B) LC-MS analysis and TIC chromatogram demonstrating the conversion of 3a to 3b with the appropriate change of mass. C) TIC chromatogram for peptide 3b synthesized using orthogonal protection is identical to the chromatogram of peptide 3b synthesized using kinetically controlled bicyclization. D) The rate of i, i+1 cyclization of peptide 4a was compared to cyclization of peptide 3a. Just 5% of peptide 4a cyclized in the first 30 seconds, while peptide 3a shows 95% cyclization in 30 seconds, indicating that the favored regioisomer for peptide 3b contains two i, i+7 cycles.
Importantly, both methods to synthesize 3b provided products with identical retention times (Figure 2C), suggesting that a bicycle with two i, i+7 macrocycles is favored over the bicycle with i, i+15 and i, i+1 macrocycles. To confirm our hypothesis that the i, i+1 reaction is slow, we synthesized the control peptide 4a containing one cysteine residue adjacent to a cysteine residue linked to decafluorobiphenyl. When treated with 50 mM DIEA in DMF, less than 5% of 4a cyclized in the first 30 seconds and less than 30% cyclized after 5 minutes (Figure 2D). In comparison, peptide 3a shows 95% conversion in 30 seconds. The slow rate for i, i+1 cyclization indicates that the favored regioisomer for peptide 3b contains two i, i+7 cycles.
Next, we tested our bicyclic compounds for their ability to promote the intracellular delivery of an 18-mer, 6 kDa PMO. The PMO-peptide conjugates were synthesized using copper-catalyzed “click” chemistry (Figure 3A). In addition to the bicyclic peptides, the PMO was conjugated to a monocyclic R12 peptide 6c, linear R12 peptide 5, a non-fluorinated bicyclic peptide 1nfb, and a recently-reported lactam-cyclized R10 peptide 7c (SI Fig. 3).[24]
Figure 3: Bicyclic peptides conjugated to PMO show increased exon-skipping activity.
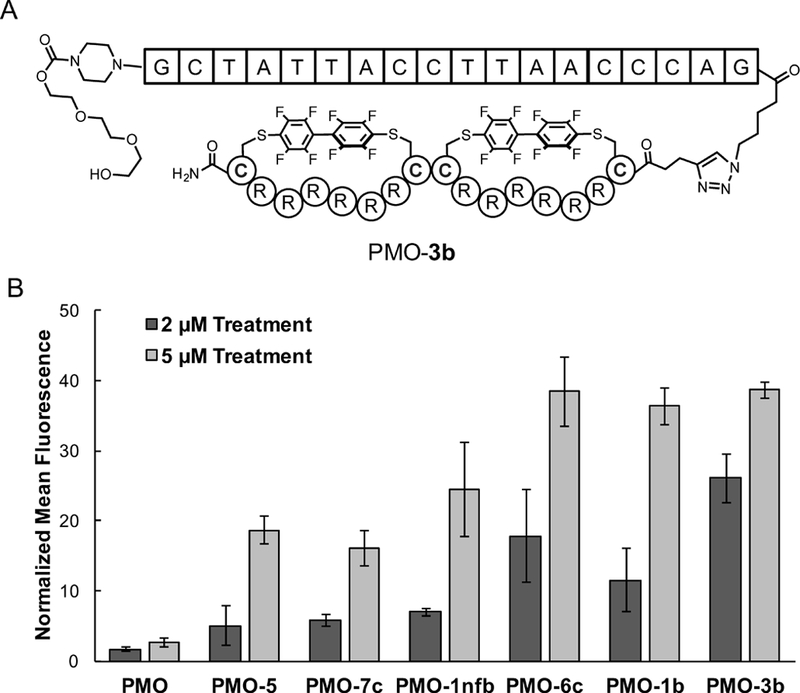
A) Depiction of bicyclic peptide conjugate PMO-3b. B) Conjugates between PMO and perfluoroaryl cyclic or bicyclic peptides (6c, 1b, and 3b) lead to more cellular fluorescence than conjugates to linear R12 (5), an established cyclic peptide cR10 (7c), or a non-fluorinated bicyclic peptide (1nfb). The PMO corrects eGFP splicing in a modified HeLa cell line. Cells were incubated with 2 μM or 5 μM of each PMO-peptide conjugate for 22 hours and the mean fluorescence intensity was analyzed by flow cytometry. Error bars are standard deviation (n=3 independent replicates).
We measured the activity of the conjugates in the HeLa-654 cell assay.[25] The HeLa-654 cells are stably transfected with an eGFP coding sequence interrupted by a mutant intron from the human β-globin gene (IVS2–654). This intron contains a mutation that alters the normal pre-mRNA splice site to a cryptic splice site, leading to retention of an unnatural exon fragment in the spliced eGFP mRNA. This aberrant mRNA leads to the translation of a non-fluorescent form of eGFP. The cargo PMO has a nucleobase sequence complementary to IVS2–654. When the PMO hybridizes to the mutant β-globin exon, it prevents the aberrant gene splicing from occurring and leads to functional eGFP expression. Successful nuclear delivery of PMO leads to the observation of green fluorescence.
To determine if bicyclic peptides improve PMO delivery, the cells were incubated with each PMO-peptide conjugate at 2 and 5 μM in media containing 10% serum. After 22 hours, cellular fluorescence was analyzed by flow cytometry (Figure 3b). At 5 μM treatment, the perfluoroaryl cyclic and bicyclic peptide conjugates all led to an approximately 14-fold increase in eGFP fluorescence relative to PMO. Both the lactam cyclized R10 and the non-fluorinated bicycle exhibited lower fluorescence, demonstrating that the perfluoroarenes contribute to the gains in PMO activity. Additionally, at 2 μM concentration, the bicyclic conjugate PMO-3b outperformed all of the other conjugates.
To investigate the role of serum, the PMO conjugates were tested in the eGFP assay using media containing 0, 5, and 10% fetal bovine serum. All conjugates exhibited only a mild reduction in activity at higher serum concentrations, suggesting that the hydrophobic perfluoroarenes are not sequestered by serum proteins (SI Fig. 4). Additionally, a lactate dehydrogenase (LDH) assay was performed to determine if these compounds disrupt the plasma membrane. For PMO-1b, LDH release was observed at both 5 μM and 2 μM, indicating early signs of cytotoxicity. At 2 μM, PMO-3b exhibited no signs of LDH release while remaining highly active (SI Fig. 5).
We examined if the increase in PMO activity resulted from structural characteristics of the peptides or solely from the combination of arginine residues with a perfluoroarene. PMO-6c was tested along with R12 variants cyclized along either the 6 N-terminal residues or the 6 C-terminal residues. The C-terminal cyclized R12 led to less eGFP fluorescence than the fully cyclic peptide 6c, demonstrating that macrocycle position affects activity (SI Fig. 6). Similarly, for the related arginine-rich peptide Bpep, the fully cyclic peptide led to more activity than the N- and C-terminal cyclic variants (SI Fig. 7). However, investigations with other peptide transporters confirmed the importance of arginine residues (SI Fig. 8).
Lastly, anticipating the peptide bicycles would exhibit enhanced stability, we measured the proteolytic stability of bicyclic versus monocyclic peptides (Figure 4). The peptides (100 μM) were incubated with trypsin (0.05 μg/mL) at 37 ºC. After one hour, less than 5% of the cyclic compound 6c remained, while 45% of bicycle 3b remained and nearly 70% of trithiol linked bicycle 1b remained. Ultimately, both bicyclic peptides demonstrated greater stability than the monocyclic peptide, and this stability could be necessary in therapeutic applications.
Figure 4: Bicyclic peptides demonstrate enhanced proteolytic stability relative to monocyclic peptides.
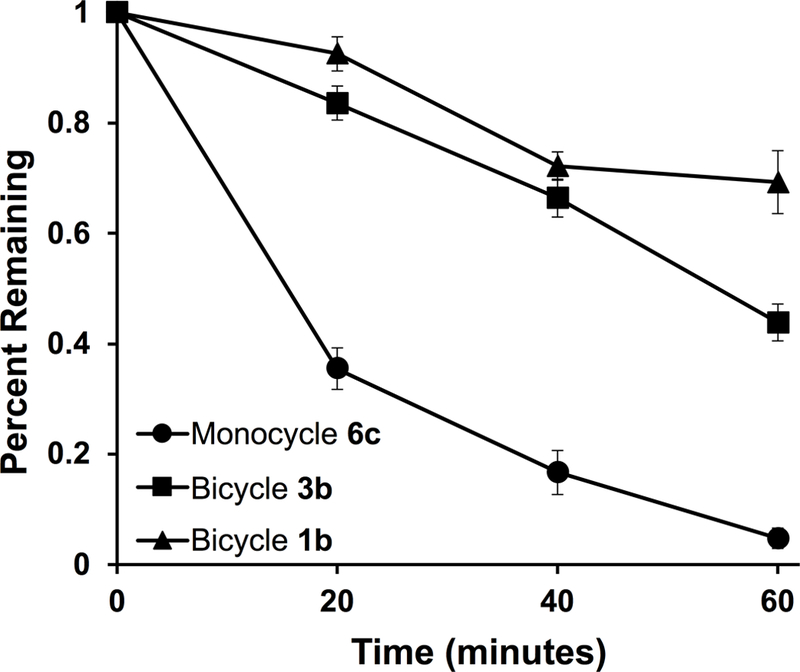
The bicyclic peptides 1b and 3b and monocyclic peptide 6c (100 μM) were incubated with trypsin (0.05 μg/mL) at 37 ºC. After 1 hour, less than 5% of 6c remained, while 45% of 3b and 70% of 1b remained, demonstrating that bicyclization improves proteolytic stability relative to monocyclization.
Here, we demonstrate multiple strategies for peptide bicyclization using cysteine SNAr chemistry. A trithiol can link three perfluoroarenes on a single peptide together into a bicycle, or two thiol pairs can be selectively crosslinked to synthesize a double macrocyclic peptide. In these studies, our cysteine arylation chemistry is shown to modulate the biological properties of arginine-rich peptides. The bicyclic peptides exhibit substantial protease resistance, and PMO-conjugated 3b does not display signs of cytotoxicity at 2 μM concentration. The exon-skipping activity of PMOs conjugated to perfluoroaryl cyclic or bicyclic peptides is greater than the activity of PMOs conjugated to other cyclic or bicyclic peptides. Moving towards in vivo applications, this control over stability, toxicity, and activity will enable the optimization of our lead compounds. Given these results, we envision that perfluoroaryl CPPs represent next-generation delivery agents for macromolecular cargo.
Supplementary Material
Table 1.
Arginine-rich peptides for bicyclization and control sequences
| Peptide | Sequence[a] | Cyclic form |
|---|---|---|
| 1 | ZCRRRRRRCRRRRRRC |
1b - Trithiol bicycle 1nfb – Non-fluorinated bicycle |
| 2 | ZCRRRCRRRC | 2b - Trithiol bicycle |
| 3 | ZCRRRRRRCCRRRRRRC | 3b - Double macrocycle |
| 4 | ZRRRRRRCCRRRRRR | 4c - Monocycle |
| 5 | ZRRRRRRRRRRRR | None |
| 6 | ZCRRRRRRRRRRRRC | 6c - Monocycle |
| 7 | XKRrRrRrRrRrE | 7c - Lactam monocycle |
Z = 4-pentynoyl, X = 5-azidopentyl; all peptides are C-terminal amides
Acknowledgements
This work was supported by Sarepta Therapeutics, Cambridge MA. J.M.W. and R.L.H are supported by the National Science Foundation Graduate Research Fellowship under Grant No. 1122374. C.M.F. is supported by the David H. Koch Graduate Fellowship Fund and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number F30HD093358.
References
- [1].Kole R, Krainer AR, Altman S, Nat. Rev. Drug Discov. 2012, 11, 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].van Roon-Mom WC, Aartsma-Rus A, in Exon Skipp. (Ed.: Aartsma-Rus A), Humana Press, 2012, pp. 79–96. [Google Scholar]
- [3].Siva K, Covello G, Denti MA, Nucleic Acid Ther. 2014, 24, 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Summerton J, Weller D, Antisense Nucleic Acid Drug Dev. 1997, 7, 187–195. [DOI] [PubMed] [Google Scholar]
- [5].Hudziak RM, Barofsky E, Barofsky DF, Weller DL, Huang S-B, Weller DD, Antisense Nucleic Acid Drug Dev. 1996, 6, 267–272. [DOI] [PubMed] [Google Scholar]
- [6].Youngblood DS, Hatlevig SA, Hassinger JN, Iversen PL, Moulton HM, Bioconjug. Chem. 2007, 18, 50–60. [DOI] [PubMed] [Google Scholar]
- [7].Li Y-F, Morcos PA, Bioconjug. Chem. 2008, 19, 1464–1470. [DOI] [PubMed] [Google Scholar]
- [8].Wu B, Li Y, Morcos PA, Doran TJ, Lu P, Lu QL, Mol. Ther. 2009, 17, 864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hanson GJ, Weller DD, Cai BZ, Zhou M, Functionally-Modified Oligonucleotides and Subunits Thereof, 2016, US9278987 B2. [Google Scholar]
- [10].Stanzl EG, Trantow BM, Vargas JR, Wender PA, Acc. Chem. Res. 2013, 46, 2944–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moulton HM, Nelson MH, Hatlevig SA, Reddy MT, Iversen PL, Bioconjug. Chem. 2004, 15, 290–299. [DOI] [PubMed] [Google Scholar]
- [12].Abes S, Moulton HM, Clair P, Prevot P, Youngblood DS, Wu RP, Iversen PL, Lebleu B, J. Controlled Release 2006, 116, 304–313. [DOI] [PubMed] [Google Scholar]
- [13].Lebleu B, Moulton HM, Abes R, Ivanova GD, Abes S, Stein DA, Iversen PL, Arzumanov AA, Gait MJ, Adv. Drug Deliv. Rev. 2008, 60, 517–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hanson GJ, Peptide Oligonucleotide Conjugates, 2015, US 9,161,948. [Google Scholar]
- [15].Timmerman P, Beld J, Puijk WC, Meloen RH, ChemBioChem 2005, 6, 821–824. [DOI] [PubMed] [Google Scholar]
- [16].Heinis C, Rutherford T, Freund S, Winter G, Nat. Chem. Biol. 2009, 5, 502–507. [DOI] [PubMed] [Google Scholar]
- [17].Chen S, Morales-Sanfrutos J, Angelini A, Cutting B, Heinis C, ChemBioChem 2012, 13, 1032–1038. [DOI] [PubMed] [Google Scholar]
- [18].Wang J, Zha M, Fei Q, Liu W, Zhao Y, Wu C, Chem. – Eur. J. n.d, n/a–n/a. [Google Scholar]
- [19].Sako Y, Morimoto J, Murakami H, Suga H, J. Am. Chem. Soc. 2008, 130, 7232–7234. [DOI] [PubMed] [Google Scholar]
- [20].Wallbrecher R, Depré L, Verdurmen WPR, Bovée-Geurts PH, van Duinkerken RH, Zekveld MJ, Timmerman P, Brock R, Bioconjug. Chem. 2014, 25, 955–964. [DOI] [PubMed] [Google Scholar]
- [21].Lian W, Jiang B, Qian Z, Pei D, J. Am. Chem. Soc. 2014, 136, 9830–9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spokoyny AM, Zou Y, Ling JJ, Yu H, Lin Y-S, Pentelute BL, J. Am. Chem. Soc. 2013, 135, 5946–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zou Y, Spokoyny AM, Zhang C, Simon MD, Yu H, Lin Y-S, Pentelute BL, Org. Biomol. Chem. 2013, 12, 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Herce HD, Schumacher D, Schneider AFL, Ludwig AK, Mann FA, Fillies M, Kasper M-A, Reinke S, Krause E, Leonhardt H, et al. , Nat. Chem. 2017, 9, 762. [DOI] [PubMed] [Google Scholar]
- [25].Sazani P, Kang S-H, Maier MA, Wei C, Dillman J, Summerton J, Manoharan M, Kole R, Nucleic Acids Res. 2001, 29, 3965–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


