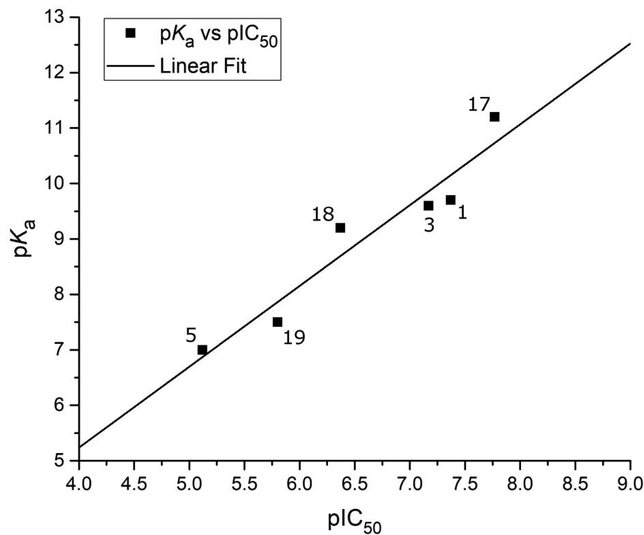
Figure 3.
Inhibition values (pIC50) of MBP isologues of compound 1 plotted against the pKa of their bridging phenolic oxygen atom. A linear correlation (r = 0.96, n = 6, p < 2×10−3) was observed between pKa and pIC50, illustrating a ligand electronics-based justification for the observed disparity in inhibition values of otherwise structurally homologous inhibitors.