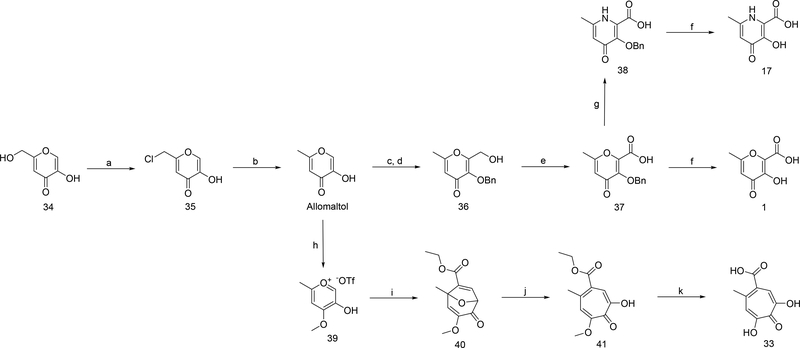
Scheme 1.
aReagents and conditions: (a) thionyl chloride, CH2Cl2, rt, 6–8 h; (b) zinc dust, HCl, water, 70 °C, 4–6 h; (c) NaOH, formaldehyde, water/MeOH, 0–20 ºC, 18 h; (d) benzyl bromide, TBACl, 70 °C, 3 h; (e) NaCO3H, KI, TEMPO, TBACl, NaOCl, CH2Cl2/water, 5 °C, 2.5 h; (f) 5:5:1 HOAc/HCl/TFA, 40 °C, 18 h. (g) NH4OH, MeOH/water, 75 °C, 18–24 h; (h) methyl triflate, DCM/chloroform, reflux, 18 h; (i) ethyl propiolate, TEA, chloroform, microwave 100 ºC, 25 min; (j) BCl3, CH2Cl2, 0–20 °C, 30 min; (k) HBr/HOAc, neat, 95 °C, 6–8 h.