Figure 1.
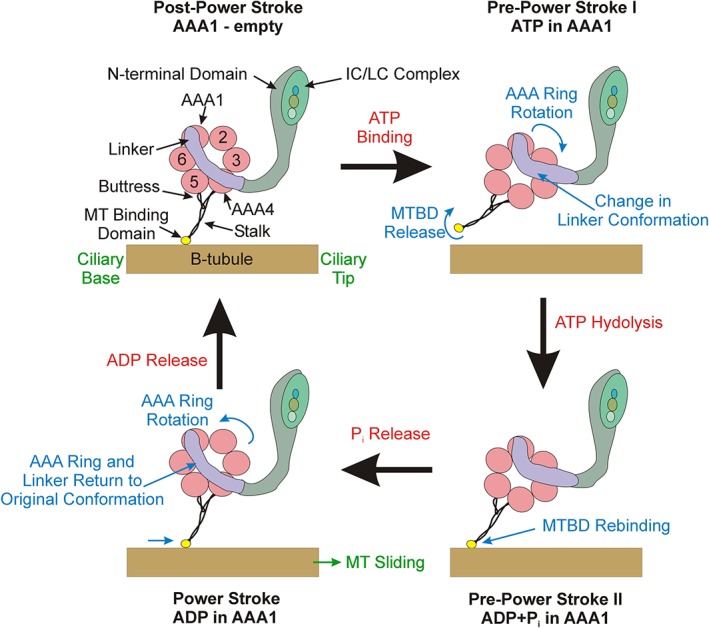
Dynein organization and power stroke structural transitions. This diagram illustrates the four major transitions that occur during the dynein mechanochemical cycle. Dynein is anchored to the A‐tubule (not shown) via the N‐terminal domain and IC/LC complex. Nucleotide binding at AAA1 converts the motor to the pre‐power stroke I conformation in which the linker (purple) undergoes an almost 90° bend causing the AAA ring to swing, simultaneously the microtubule‐binding domain (MTBD) adopts a low affinity conformation and releases the B‐tubule. Following nucleotide hydrolysis, the MTBD transitions to a high affinity state and rebinds the microtubule at a new site. Phosphate release triggers the return of the linker to its original unbent state and thus drives the attached microtubule toward the axonemal distal tip. Finally, ADP departs the active site leaving the AAA1 nucleotide pocket empty and the HC capable of undergoing another mechanochemical cycle. HC, heavy chain; IC, intermediate chain; LC, light chain [Color figure can be viewed at http://wileyonlinelibrary.com]
