Abstract
Objective:
To determine the incidence of and risk factors for development of celiac disese (CD) in individuals with type 1 diabetes.
Methods:
Cohort study using The Health Improvement Network (THIN), a United Kingdom primary care database of >13 million people. Individuals with incident type 1 diabetes diagnosed at 1–35 years of age between 1995 and 2015 with no previous diagnosis of CD were included. Cox regression was used to identify risk factors for CD, including age at diabetes diagnosis and sex, while adjusting for year of diagnosis to control for potential rising incidence in CD over time.
Results:
Subjects (n=9,180; 43% female) had a median observation time of 5.1 years (IQR 2.0–10.1). CD was diagnosed in 196 (2%) during follow up. Median time to diagnosis was 2.1 years, but 25% were diagnosed more than 5 years after diabetes diagnosis. Incidence (per 10,000 person-years) was greater in females (43.0 [95%CI 35.2–52.0]) vs males (26.8 [95%CI 21.5–32.9]). In multivariable Cox regression stratified by childhood- versus young adult-onset diabetes, younger age at diabetes diagnosis within childhood (HR 0.91 [95% CI 0.88–0.94]) and female sex among the adult-onset diabetes group (HR 3.19 [95% CI 1.39–7.34]) were associated with greater risk of CD.
Conclusions:
As expected, incidence of CD was higher in individuals with childhood-onset diabetes versus those with adult-onset diabetes. However, individuals with diabetes are at risk of developing CD throughout childhood and adulthood, and prolonged screening after diagnosis may be warranted. Prospective studies are needed in order to guide risk-stratified approaches to screening.
Keywords: celiac disease, type 1 diabetes, screening, epidemiology, incidence
Introduction
Celiac disease (CD), an enteropathy due to gluten sensitivity in genetically susceptible individuals 1, occurs at higher rates among individuals with type 1 diabetes than the general population.2 CD is associated with significant morbidity, including anemia, osteoporosis, short stature, chronic fatigue, peripheral neuropathy or ataxia, and gastrointestinal lymphoma.3 Among patients with diabetes, symptomatic hypoglycemia has been linked to untreated CD, with noted improvement after institution of a gluten-free diet.3 Notably, the risk of mortality in patients with diabetes increases with longer duration of CD.4 Early diagnosis and treatment of CD in patients with diabetes may therefore improve clinical outcomes and allow for earlier indentification and managment of potential CD-related complications.5,6
Because CD may be asymptomatic, a high index of suspicion or a screening protocol is necessary for diagnosis in individuals with diabetes.2,7 The International Society for Pediatric and Adolescent Diabetes recommends routine CD screening every 1–2 years,8 but evidence for screening beyond the first five years of diagnosis is lacking.2 Other leading organizations, including the United Kingdom’s National Institute for Health and Clinical Excellence (NICE) acknowledge the lack of evidence to determine the appropriate screening interval or duration.9,10 Although several studies have demonstrated increased risk of CD among children who were younger at diabetes diagnosis 11–16, no modifications to screening based on age are suggested by any existing guidelines. In addition, although approximately one-quarter to nearly one-half of patients with diabetes are diagnosed as adults 17,18, few studies have evaluated CD risk factors or incidence among adults with diabetes. Related to the lack of routine screening, CD diagnosis in adults with diabetes may be significantly delayed.19 An additional potential risk factor for CD, female sex, has been variably shown to increase risk of CD in children with diabetes.2,13,15 Sex may have a differential impact on CD risk in patients with diabetes depending on age, but to our knowledge, this potential interaction has not been systematically evaluated over a large age range spanning childhood and adulthood.
In this population-based cohort study, we sought to determine the incidence of CD in children and young adults diagnosed with diabetes over a period of two decades and to describe the impact of age at diabetes diagnosis and sex on CD diagnosis.
Research Design and Methods
A. Data Source
The observational data used in this retrospective cohort study was obtained from The Health Improvement Network (THIN) database, an anonymized longitudinal primary care electronic medical records database from the United Kingdom (UK). THIN currently includes over 13 million patients, representing approximately 5–6% of the UK population. THIN contains demographics, diagnoses and procedures (as recorded by the general practioner using Read codes, the standard classification system in the UK), laboratory data, and prescription records of participating practices.20 Data collected from 1995 to 2015 were used for this analysis. Read codes for diabetes and celiac disease are provided in Supporting Information Table 1a. THIN has been used to characterize the risk of fracture in children and adults with type 1 diabetes, demonstrating the ability to capture our desired cohort of patients with diabetes.21 Access to THIN was made available to the authors through an agreement with The University of Pennsylvania.
B. Study Cohort
The study cohort consisted of patients who were diagnosed with diabetes between 1 and 35 years of age. We included only diagnoses of diabetes after the first 90 days of patient registration with the participating practice (incident diabetes) in order to minimize the possibility of misclassification bias from retroactively recorded historical diagnoses 22 and to allow for time-to-event analysis. We included all individuals who had at least one specific Read code consistent with type 1 diabetes. We also included individuals who had at least one non-type-specific diabetes code (Supporting Information Table 1a) and a prescription for insulin in the year following the first diabetes code but no prescription for oral or other injectable hypoglycemic medications in the year following the first diabetes code (medication codes in Supporting Information Table 1b). Individuals with non-type-specific diabetes codes and other Read codes for type 2 diabetes, gestational diabetes, neonatal diabetes, maturity onset diabetes of the young (MODY), cystic fibrosis-related diabetes, or steroid-induced diabetes were excluded. We excluded individuals who were 35 years or older at the time of diabetes diagnosis to reduce misclassification bias when using non-type-specific diabetes codes.23 To minimize misclassification of neonatal (monogenic) diabetes as autoimmune type 1 diabetes, we excluded individuals less than 1 year of age at first diabetes code. We excluded individuals whose birth, registration, or death or transfer dates were out of sequence (e.g. birth date after registration date). No diabetes-related serology is available in the dataset.
C. Follow-up
Follow-up for CD-free survival began at the date of the first diabetes Read code (index date). The endpoint was CD diagnosis, and individuals were censored if they transferred out of their practice or died, or the practice stopped collecting data.
D. Primary Outcome
The primary outcome of interest was incident diagnosis of CD. Incident CD was defined as at least one Read code consistent with CD (Coeliac disease J690.00, Coeliac disease NOS 690z00, Coeliac disease autoantibody profile positive 68W4.00, Gluten enteropathy J690.13, Dietary advice for coeliac disease ZC2C200) occurring at least one day after diabetes diagnosis date. Although CD serology values (e.g. tissue transglutaminase, anti-endomysial antibody, anti-gliadin) are not included in the database, THIN has been used to study pediatric and adult celiac disease 24–26, and the use of Read codes for CD diagnosis was previously validated.27
E. Covariates
Age at diabetes diagnosis and sex were of primary interest as covariates in the Cox regression models. Additional covariates included other conditions of autoimmunity associated with diabetes, including hypothyroidism, hyperthyroidism, and adrenal insufficiency; these were included as time-varying covariates. These conditions were identified by Read code (Supporting Information Table 1a). Year of diabetes diagnosis was analyzed to adjust for secular trends in CD incidence. Body mass index (BMI) and hemoglobin A1c were also obtained for descriptive analyses but were not included in the Cox regression due to high rates of missingness.
F. Statistical Analysis
For descriptive statistics, categorical variables were reported as proportions, and continuous variables were summarized using median and interquartile range (IQR) because the variables assessed were not normally distributed. Time-to-event analysis using Cox proportional hazards models was performed to evaluate potential risk factors for CD diagnosis. All covariates of interest were included in the initial multivariable Cox regression model and sequentially eliminated until only covariates with p-value <0.05 remained (backward elimination). The proportional hazards assumption was tested using Schoenfeld residuals and graphically using log-log plots. Kaplan-Meier curves were generated for CD-free survival, and differences in CD-free survival by sex and age at diabetes diagnosis were compared via the log-rank test.
Analyses were performed using Stata 14 (StataCorp LP, College Station, TX). The study protocol was reviewed and approved by the THIN Scientific Review Committee. It was reviewed by the University of Pennsylvania and the Children’s Hospital of Philadelphia Institutional Review Boards (IRB) and determined to meet eligibility criteria for IRB exemption authorized by 45 CFR 46.101, category 4 and 45 CFR 102(f).
G. Sensitivity analyses
To investigate the possibility of bias related to misclassification of diabetes diagnosis, a sensitivity analysis was performed in which we restricted the cohort to only individuals with at least one Read code specific for type 1 diabetes. To investigate the possibility of bias related to misclassification of CD diagnosis, a second sensitivity analysis was performed in which individuals with the Read code “Coeliac disease autoantibody profile positive 68W4.00” as the only CD-related Read code were included as non-CD cases. Finally, to minimize the possibility of changing disease definitions due to evolving testing strategies for CD, including different assays and screening practices, a third sensitivity analysis restricted the cohort to only the latter 10 years, 2005–2015.
Results
A. Cohort characteristics
We identified 9,228 individuals younger than 35 years of age at the time of diabetes diagnosis; 45 (0.5%) individuals had a prior diagnosis of CD, and 3 were diagnosed with both CD and diabetes on the same date. Of the 9,180 individuals with incident diabetes without prevalent or concurrent CD, 8,293 (90.3%) had Read codes specific for type 1 diabetes, and 887 (9.7%) had non-type-specific codes for diabetes and met the added inclusion criteria of insulin-dependence without other hypoglycemic medication use in the first year of diabetes diagnosis. 4,558 (49.7%) individuals were diagnosed with diabetes in childhood (< 18 years of age). Females comprised 43% of the entire cohort, but the proportion differed between adult-onset and childhood-onset diabetes (45% female in childhood-onset versus 42% in adult-onset diabetes, p = 0.01 by chi-square test). Of note, a male predominance in type 1 diabetes has been previously demonstrated, particularly among European cohorts,28,29 including in the United Kingdom.30
Table 1 lists subject characteristics. The median age at diabetes diagnosis was 18.2 years (IQR 10.7–27.5, range 1–35). Of the 196 (2.1%) individuals diagnosed with incident CD, median time to CD diagnosis was 2.1 years (IQR 1.0–5.0); 25% were diagnosed more than 5 years after diabetes diagnosis. The vast majority (188/196, 96%) of identified CD cases had at least one CD-specific Read code (Coeliac disease J690.00), and only 8 subjects had only a CD-antibody related code (Coeliac disease autoantibody profile positive 68W4.00). The median observation time was 5.1 years (IQR 2.0–10.1), with total follow-up time of 58,265 person-years. Median average hemoglobin A1c was 8.6% (70.7 mmol/mol; IQR 7.7–10.0% [60.6–85.3 mmol/mol]) in the 5,646 individuals with at least one HbA1c available for analysis during the observation period and did not differ significantly between individuals with and without incident CD (p>0.5 by Wilcoxon rank-sum test). 584 individuals (6%; 65% female), had a diagnosis of thyroid disease (hypo- or hyperthyroidism), and 16 (0.2%; 38% female) had a diagnosis of adrenal insufficiency. Four individuals had both thyroid disease and adrenal insufficiency.
Table 1—
Cohort characteristics, median (IQR) or n (%)
| N (% female) | 9,180 (43%) |
| Patient-years of follow-up | 58,265 |
| Age at diabetes Diagnosis (years) | 18.2 (10.7–27.5) |
| Year at diabetes Diagnosis | 2005 (2001–2010) |
| Follow-up time (years) | 5.1 (2.0–10.1) |
| Celiac disease | 196 (2.1%) |
| Time to CD diagnosis (years) | 2.1 (1.0–5.0) |
| Hypothyroidism | 467 (5.1%) |
| Hyperthyroidism | 113 (1.2%) |
| Thyroid disease (non-specific) | 4 (0.04%) |
| Adrenal insufficiency | 16 (0.2%) |
| Average hemoglobin A1c (% [mmol/mol]) | 8.6% (7.7–10.0%) [70.7 (60.6–85.3)] |
| Body mass index (kg/m2) | 23.5 (19.8–27.5) |
B. Celiac disease incidence by age and sex
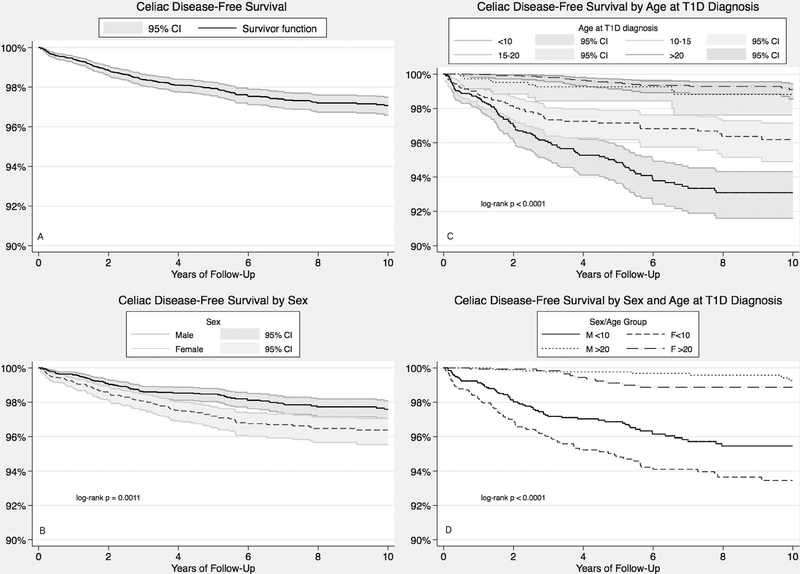
The incidence of CD in the cohort was 33.6 (95% CI 29.1–38.7) per 10,000 person-years and was greater in females than males (43.0 [95% CI 35.2–52.0] vs 26.8 [95% CI 21.5–32.9] per 10,000 person-years) (Table 2). Incidence was greater with childhood-onset (<18 years) than young adult-onset (≥18 years) diabetes (57.9 [95% CI 49.5–67.2] vs 9.0 [95% CI 5.9–13.2] per 10,000 person-years). Kaplan-Meier curves representing CD-free survival over time are depicted in Figure 1, including CD-free survival distribution for the overall cohort (Figure 1A). CD-free survival was significantly lower for females (Figure 1B, p < 0.001 by log-rank test) and individuals who were younger at the time of diabetes diagnosis (Figure 1C, p = 0.001 by log-rank test for age categories of 1–10, 10–15, 15–20, and 20–35 years). Figure 1D demonstrates the difference in CD-free survival across combined age- and sex-categories, depicting distributions for the youngest (< 10 years) and oldest (>20 years) individuals at diabetes diagnosis (p < 0.0001 by log-rank test).
Table 2—
Incidence per 10,000 person-years (95% CI) of CD
| Age at Follow-up (years) | Sex | |||||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| CD Cases | Person-Years | Incidence | CD Cases | Person-Years | Incidence | |
| 1–10 | 10 | 3014 | 33 (16–61) | 13 | 2640 | 49 (26–84) |
| 10–20 | 42 | 10201 | 41 (30–56) | 57 | 7938 | 72 (54–93) |
| 20–30 | 29 | 8847 | 33 (22–47) | 21 | 6059 | 35 (21–53) |
| 30–40 | 4 | 8823 | 4.5 (1.2–12) | 7 | 6223 | 11 (4.5–23) |
| 40+ | 5 | 2745 | 18 (5.9–43) | 8 | 1775 | 45 (19–89) |
| Overall | 90 | 33631 | 26.8 (21.5–32.9) | 106 | 24634 | 43.0 (35.2–52.0) |
Figure 1—
Kaplan-Meier curves of CD-free survival over 10 years of follow-up, depicting overall cohort (A) and stratified by (B) sex, (C) age at diabetes diagnosis, and (D) both sex and age at diabetes diagnosis. Survival distributions differed significantly across strata in each analysis (p<0.05 by log-rank test).
C. Factors associated with increased hazard of celiac disease
In multiivariable Cox regression, sex, age at diabetes diagnosis, year of diabetes diagnosis, and adrenal insufficiency remained significantly associated with CD (p<0.05) and were included in the final model. Thyroid disease was eliminated from the model due to lack of significance (HR 1.36, 95% CI 0.69–2.68, p = 0.38). Age of diabetes diagnosis violated the proportional hazards assumption. Due to significant interactions between age and sex, we stratified our analysis by childhood-onset (<18 years, n = 4,558) versus adult-onset (≥18 years, n = 4,622) diabetes. Using the clinically-relevant age threshold of 18 years, the proportional hazards assumption was no longer violated within strata.
Adjusted for year of diabetes diagnosis, the impact of age at diagnosis and sex differed by strata, as shown by the adjusted HRs in Table 3. Hazard of CD was significantly greater for diabetes diagnosis at younger age within childhood (HR 0.91 [95% CI 0.88–0.94], by year of age) but was not significantly impacted by sex (HR 1.31 [95% CI 0.97–1.77]). Adrenal insufficiency was statistically significantly associated with CD, but the estimate of the hazard ratio had very poor precision (HR 15.2 [95% CI 2.1–110.0]) due to the few individuals with this diagnosis. Among individuals diagnosed with diabetes in adulthood, hazard of CD was greater for female sex (HR 3.19 [95% CI 1.39–7.34]). Although the incidence of CD was lower among subjects with adult-onset diabetes than childhood-onset, age was no longer a significant risk factor within the adult-onset group (HR 1.01 [95% CI 0.93–1.10]). Adrenal insufficiency was not a significant risk factor among individuals diagnosed with diabetes in adulthood.
Table 3—
Multivariable Cox regression for celiac disease
| Adjusted HR (95% CI) | ||
|---|---|---|
| Covariate | < 18 years at diabetes diagnosis | ≥18 years at diabetes diagnosis |
| Female (ref: male) | 1.31 (0.97–1.77) | 3.19 (1.39–7.34)* |
| Age at diabetes diagnosis (years) | 0.91 (0.88–0.94)* | 1.01 (0.93–1.10) |
| Year of diabetes diagnosis | 1.05 (1.02–1.08)* | 1.06 (0.96–1.17) |
| Adrenal insufficiency (time-varying) | 15.19 (2.10–110.00)* | |
| Person-years (n) | 29,380 (4,558) | 28,884 (4,622) |
p<0.01
D. Sensitivity analyses
To test the strength of associations found in the main multivariable Cox regression analysis, the cohort was restricted to only individuals with diabetes-specific Read codes. This reduced the size of the cohort to 8,293 with 192 cases of CD, but strength and significance of each association was nearly identical (Supplemental Table 2). To minimize the potential for misclassification of the outcome, individuals with the Read code “Coeliac disease autoantibody profile positive 68W4.00” as the only CD-related Read code were included as non-CD cases, limiting the CD cases to 188. Again, the strength and signfiicance of each association was nearly identical (Supplemental Table 3). Finally, to allow for a more consistent disease definition of CD, we limited the analysis to 2005–2015 only. The overall incidence (48.9 per 10,000 person-years, 95% CI 39.4–59.9) was higher than that for the original cohort, but the greater incidence in females (69.3 per 10,000, 95% CI 52.2–90.2) versus males (34.0 per 10,000, 23.9–46.8) persisted. In multivariable Cox regression, age no longer violated proportional hazards, so no stratification by age at diabetes diagnosis was necessary. Female sex remained a significant risk factor with an intermediate HR compared to the stratified analyses (HR 1.81, 95% CI 1.19–2.74), and age was protective, with a HR similar to that for the childhood-onset stratum (HR 0.89, 95% CI 0.87–0.92). Within this limited time frame, year of diagnosis was no longer significant in the model, and adrenal insufficiency lost significance due to sample size (Supplemental Table 4).
Discussion
This large, population-based cohort study of children and young adults found that risk of celiac disease among patients with diabetes is significantly greater for those diagnosed with diabetes at younger ages, independent of sex or time period of diagnosis. In addition, we demonstrate a greater hazard of CD in women, but only for those with diabetes diagnosed in young adulthood. Incidence of CD in our cohort was more than 20-fold greater than among the general UK population in the same time period (0.5–1.9 per 10,000 person-years),24 which is evidence in support of screening in this high-risk population.
Our finding that 25% of individuals were diagnosed with CD more than five years after diabetes diagnosis suggests the potential value of prolonged routine screening for the highest-risk individuals (those diagnosed in early childhood). Routine screening of asymptomatic children may be especially valuable given the negative impact of untreated CD on bone mineral density and the critical period of bone accrual during childhood. Our finding of increased risk of CD with younger-onset diabetes is in agreement with previous studies of children with diabetes.2,12,13,15 The mechanism underlying increased risk of CD with younger age at diabetes diagnosis may be related to the hypothesis of common determinants for both diseases 13, including environmental exposures, genetic background (including HLA and non-HLA variants) 31,32, and the gut microbiome.33 Combined exposure history and genetic predisposition may impact autoimmunity: for example, timing of cereal introduction to infants genetically susceptible to diabetes or CD is associated with seropositivity, and certain viral infections have been linked to diabetes and CD risk.33 To explore the hypothesis of common determinants of autoimmune disease, we evaluated the impact of autoimmune thyroid disease and adrenal insufficiency on CD diagnosis over time. Although thyroid disease was not associated with greater hazard of CD, this finding likely reflects the overall high prevalence of thyroid disease in individuals with diabetes.34,35 Our finding of increased risk of CD after diagnosis of adrenal insufficiency is clinically plausible, but the precision around our estimate was limited due to the rarity of the diagnosis. Our finding of increased risk of CD diagnosis in adult females aligns with the sex differences in CD incidence in the general population.25 The differential impact of sex with age at diabetes diagnosis may be related to differences in sex hormone exposure with age. Alternatively, the overall greater risk of CD in childhood may outweigh any sex-specific effects that are detectable in the adult population.
Our study has several strengths, including the large cohort of both children and young adults with diabetes that is population-based, increasing the generalizability of our findings. Previous studies assessing CD risk in children with diabetes have had limited to no follow-up into adulthood.2,11,13,15,36 An additional strength of our study was our use of time-to-event analysis to assess the impact of both age at diabetes diagnosis and duration of disease. This analysis allows for an improved understanding of potential risk factors while adjusting for time-varying covariates and accounting for secular trends in CD diagnosis over time. Our study adds to the recent large, multinational cross-sectional study that included youth in the United Kingdom National Pediatric Diabetes Audit by Craig and Prinz et al.15 Due to the longitudinal follow-up available through THIN, our study allowed for assessment of incidence of CD into adulthood and among young adults newly diagnosed with diabetes.
Two potential biases were possible due to the design of our study: misclassification bias and ascertainment bias. Although we were unable to validate the diagnosis of diabetes using diabetes autoantibodies, our age restriction to diabetes diagnosis less than 35 years was conservative and in line with that suggested by Royal College of General Physicians to reduce misclassification bias when using non-type-specific diabetes codes.23 Our consistent findings when we restricted the cohort to only individuals with type 1 diabetes-specific Read codes suggests that no significant misclassification bias for diabetes occurred. We acknowledge that additional hypoglycemic medication use by individuals with type 1 diabetes is becoming more common and so it is possible that by excluding patients who were prescribed oral hypoglycemic drugs we may have inadvertently excluded some subjects with Type I diabetes from our cohort. This would have the effect of overestimating the incidence of CD. However, we were more concerned about incorrectly including patients with Type 2 diabetes, which would have led to an underestimation of the incidence of CD in patients with Type I diabetes.
Misclassification of the outcome is also possible due to our inability to confirm CD diagnoses with serology or biopsy results. This could result in over-estimation of risk due to the potential for normalization of antibody levels in individuals with recently-diagnosed type 1 diabetes even while continuing a gluten-containing diet.37 However, the accuracy of CD diagnosis using Read codes recorded electronically by UK general practioners was previously validated, with a positive predictive value of 81% for a single code.24,27 In addition, our prevalence estimates are in line with previous studies of patients with diabetes 2 and reflect a lower-range estimate of true prevalence due to inclusion of only individuals who were diagnosed with diabetes during follow-up and those without a diagnosis of CD that preceded diabetes. Notably, our estimated 4.1% prevalence of CD among children with diabetes (105 CD cases among 2,585 children younger than 18 years) was very close to the 3.8% prevalence reported by Craig and Prinz et al (645 cases of CD among 17,152 individuals).15 It is possible that our findings reflect ascertainment bias, but this is less likely than among the general population due to routine screening. Recommendations to screen for CD in patients with diabetes at diagnosis and every 3 years subsequently were put forth in 2004 by the United Kingdom’s National Institute of Health and Clinical Excellence (NICE), but physicians within the UK have advocated for annual screening in individuals with diabetes.38,39 In addition, we found the same significant associations with age and sex in a sensitivity analysis restricting the cohort to those diagnosed with diabetes in the last 10 years of our study. However, prospective, longitudinal studies throughout childhood and adulthood would be needed to determine the true incidence of asymptomatic CD. Alternatively, large retrospective datasets that include details about screening, both ordered and obtained, may reduce potential ascertainment bias that could result from preferentially testing youth or females, for example. However, these screening data are often only available in institutional cohorts, and these studies would not benefit from the large sample size and power available in this study. Finally, our conclusions are limited to the United Kingdom due to the recognized geographical and ethnic variation in incidence and prevalence of CD.15,40
In summary, we found that younger age at diabetes independently increased the risk of incident CD in this cohort of both children and adults with diabetes, even after adjusting for time period of diagnosis to account for increasing incidence with time. Female sex was associated with an increased hazard of CD only for those diagnosed with diabetes in young adulthood. Our novel finding that 25% of individuals were diagnosed with CD greater than 5 years from diabetes diagnosis, coupled with the recognition of the difference in risk by age and sex, suggests that a more individualized approach to screening may be warranted. Specifically, regular screening beyond the first 5 years of diagnosis may be warranted for individuals diagnosed in early childhood.
Supplementary Material
Acknowledgments.
The authors acknowledge Qufei Wu, MS (Biostatistics Analysis Center, University of Pennsylvania) for his help with data extraction.
This work was support by National Institute of Diabetes and Digestive and Kidney Diseases grants T32-DK07314 (MEV), K23-DK-114477 (DRW), and K23-DK-093556 (MRD) as well as a grant from the Endocrine Fellows Foundation (MEV). MEV is a fellow of the Center for Healthcare Improvement and Patient Safety and the Leonard Davis Institute of Health Economics at the University of Pennsylvania.
The authors receive(d) funding from the following sponsors for research outside the submitted work: Mallinckrodt Pharmaceuticals unrelated to this work (MRD). The authors have no other potential conflicts of interest relevant to report.
Abbreviations
- BMI
body mass index
- CD
celiac disease
- HR
hazard ratio
- IQR
interquartile range
- NICE
United Kingdom’s National Institute for Health and Clinical Excellence
- THIN
The Health Improvement Network database
References
- 1.Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357(17):1731–1743. [DOI] [PubMed] [Google Scholar]
- 2.Pham-Short A, Donaghue KC, Ambler G, Phelan H, Twigg S, Craig ME. Screening for Celiac Disease in Type 1 Diabetes: A Systematic Review. Pediatrics. 2015;136(1):e170–176. [DOI] [PubMed] [Google Scholar]
- 3.Green PHR. The many faces of celiac disease: Clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128(4):S74–S78. [DOI] [PubMed] [Google Scholar]
- 4.Mollazadegan K, Sanders DS, Ludvigsson J, Ludvigsson JF. Long-term coeliac disease influences risk of death in patients with type 1 diabetes. J Intern Med. 2013;274(3):273–280. [DOI] [PubMed] [Google Scholar]
- 5.Lau MS, Sanders DS. Optimizing the diagnosis of celiac disease. Curr Opin Gastroenterol. 2017;33(3):173–180. [DOI] [PubMed] [Google Scholar]
- 6.Simmons KM, McFann K, Taki I, et al. Reduced Bone Mineral Density Is Associated with Celiac Disease Autoimmunity in Children with Type 1 Diabetes. J Pediatr. 2016;169:44–48 e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bottaro G, Cataldo F, Rotolo N, Spina M, Corazza GR. The clinical pattern of subclinical/silent celiac disease: an analysis on 1026 consecutive cases. Am J Gastroenterol. 1999;94(3):691–696. [DOI] [PubMed] [Google Scholar]
- 8.Kordonouri O, Klingensmith G, Knip M, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Other complications and diabetes-associated conditions in children and adolescents. Pediatr Diabetes. 2014;15 Suppl 20:270–278. [DOI] [PubMed] [Google Scholar]
- 9.Richey R, Howdle P, Shaw E, Stokes T, Guideline Development G. Recognition and assessment of coeliac disease in children and adults: summary of NICE guidance. BMJ. 2009;338:b1684. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of Medical Care in Diabetes—2017. Diabetes Care. 2017. [Google Scholar]
- 11.Pham-Short A, Donaghue KC, Ambler G, Chan AK, Craig ME. Coeliac disease in Type 1 diabetes from 1990 to 2009: higher incidence in young children after longer diabetes duration. Diabet Med. 2012;29(9):e286–289. [DOI] [PubMed] [Google Scholar]
- 12.Hansen D, Brock-Jacobsen B, Lund E, et al. Clinical benefit of a gluten-free diet in type 1 diabetic children with screening-detected celiac disease: a population-based screening study with 2 years’ follow-up. Diabetes Care. 2006;29(11):2452–2456. [DOI] [PubMed] [Google Scholar]
- 13.Cerutti F, Bruno G, Chiarelli F, et al. Younger age at onset and sex predict celiac disease in children and adolescents with type 1 diabetes: an Italian multicenter study. Diabetes Care. 2004;27(6):1294–1298. [DOI] [PubMed] [Google Scholar]
- 14.Kaspers S, Kordonouri O, Schober E, et al. Anthropometry, metabolic control, and thyroid autoimmunity in type 1 diabetes with celiac disease: A multicenter survey. J Pediatr. 2004;145(6):790–795. [DOI] [PubMed] [Google Scholar]
- 15.Craig ME, Prinz N, Boyle CT, et al. Prevalence of Celiac Disease in 52,721 Youth With Type 1 Diabetes: International Comparison Across Three Continents. Diabetes Care. 2017;40(8):1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianchi M, Cartabia M, Clavenna A, et al. Serological screening for celiac disease in a northern Italian child and adolescent population after the onset of type 1 diabetes: a retrospective longitudinal study of a 7-year period. Eur J Gastroenterol Hepatol. 2016;28(6):696–701. [DOI] [PubMed] [Google Scholar]
- 17.Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr Clin North Am. 2005;52(6):1553–1578. [DOI] [PubMed] [Google Scholar]
- 18.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. The Lancet Diabetes & Endocrinology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakker SF, Tushuizen ME, Stokvis-Brantsma WH, et al. Frequent delay of coeliac disease diagnosis in symptomatic patients with type 1 diabetes mellitus: clinical and genetic characteristics. Eur J Intern Med. 2013;24(5):456–460. [DOI] [PubMed] [Google Scholar]
- 20.THIN Database. https://www.ucl.ac.uk/pcph/research-groups-themes/thin-pub/database.
- 21.Weber DR, Haynes K, Leonard MB, Willi SM, Denburg MR. Type 1 diabetes is associated with an increased risk of fracture across the life span: a population-based cohort study using The Health Improvement Network (THIN). Diabetes Care. 2015;38(10):1913–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf. 2005;14(7):443–451. [DOI] [PubMed] [Google Scholar]
- 23.Royal college of general practitioners and NHS Diabetes. Coding, classification and diagnosis of diabetes. 2011. [Google Scholar]
- 24.West J, Fleming KM, Tata LJ, Card TR, Crooks CJ. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol. 2014;109(5):757–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zingone F, West J, Crooks CJ, et al. Socioeconomic variation in the incidence of childhood coeliac disease in the UK. Arch Dis Child. 2015;100(5):466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhalwani NN, West J, Sultan AA, Ban L, Tata LJ. Women with celiac disease present with fertility problems no more often than women in the general population. Gastroenterology. 2014;147(6):1267–1274 e1261; quiz e1213–1264. [DOI] [PubMed] [Google Scholar]
- 27.West J Coeliac disease: studies of its frequency and consequence PhD thesis, University of Nottingham; 2005. [Google Scholar]
- 28.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blohme G, Nystrom L, Arnqvist HJ, et al. Male predominance of type 1 (insulin-dependent) diabetes mellitus in young adults: results from a 5-year prospective nationwide study of the 15–34-year age group in Sweden. Diabetologia. 1992;35(1):56–62. [DOI] [PubMed] [Google Scholar]
- 30.UK D Diabetes: Facts and Stats. United Kingdom; 2014. [Google Scholar]
- 31.Gutierrez-Achury J, Romanos J, Bakker SF, et al. Contrasting the Genetic Background of Type 1 Diabetes and Celiac Disease Autoimmunity. Diabetes Care. 2015;38 Suppl 2:S37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagopian W, Lee HS, Liu E, et al. Co-occurrence of Type 1 Diabetes and Celiac Disease Autoimmunity. Pediatrics. 2017;140(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohn A, Sofia AM, Kupfer SS. Type 1 diabetes and celiac disease: clinical overlap and new insights into disease pathogenesis. Curr Diab Rep. 2014;14(8):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triolo TM, Armstrong TK, McFann K, et al. Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care. 2011;34(5):1211–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker JM. Clinical review: Type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab. 2006;91(4):1210–1217. [DOI] [PubMed] [Google Scholar]
- 36.Barera G, Bonfanti R, Viscardi M, et al. Occurrence of celiac disease after onset of type 1 diabetes: a 6-year prospective longitudinal study. Pediatrics. 2002;109(5):833–838. [DOI] [PubMed] [Google Scholar]
- 37.Waisbourd-Zinman O, Hojsak I, Rosenbach Y, et al. Spontaneous normalization of anti-tissue transglutaminase antibody levels is common in children with type 1 diabetes mellitus. Dig Dis Sci. 2012;57(5):1314–1320. [DOI] [PubMed] [Google Scholar]
- 38.Jones HJ, Warner JT. NICE clinical guideline 86. Coeliac disease: recognition and assessment of coeliac disease. Arch Dis Child. 2010;95(4):312–313. [DOI] [PubMed] [Google Scholar]
- 39.Babiker A, Morris MA, Datta V. Coeliac disease and type 1 diabetes: 7 years experience versus NICE guidance 2009. Arch Dis Child. 2010;95(12):1068–1069. [DOI] [PubMed] [Google Scholar]
- 40.Kang JY, Kang AH, Green A, Gwee KA, Ho KY. Systematic review: worldwide variation in the frequency of coeliac disease and changes over time. Aliment Pharmacol Ther. 2013;38(3):226–245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.