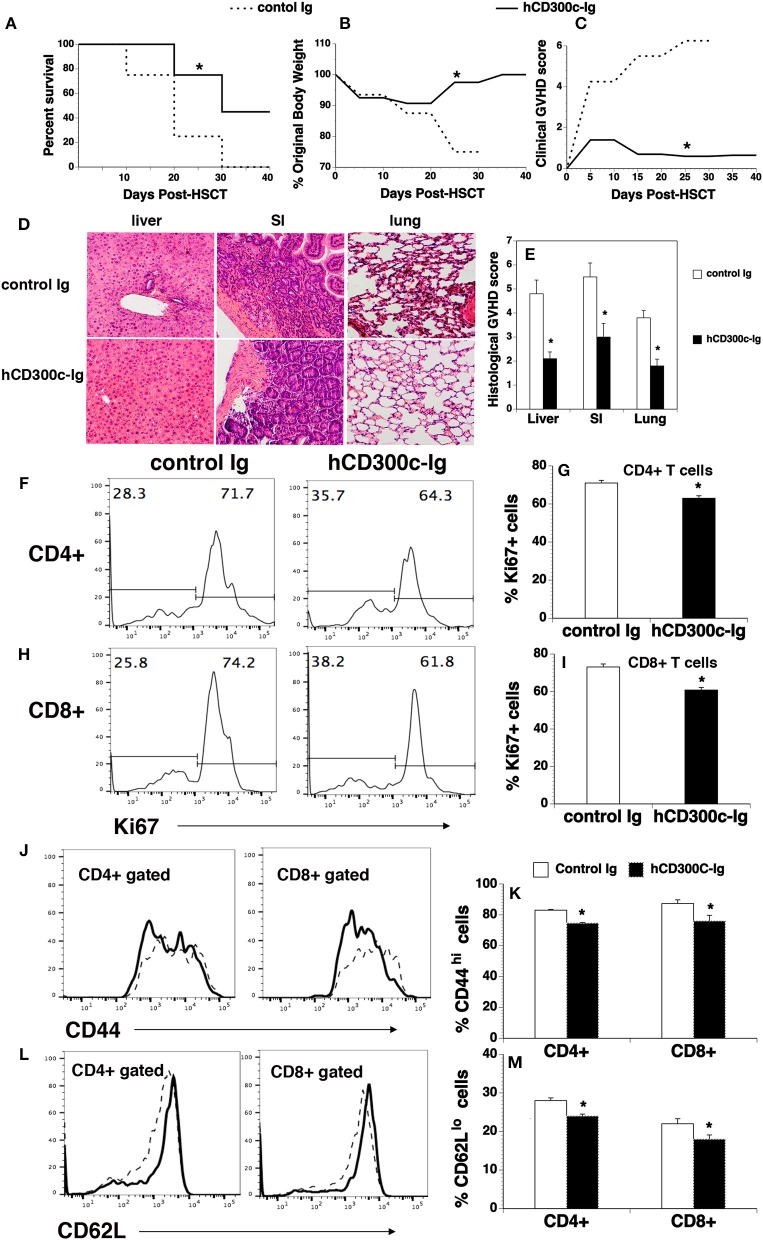
Figure 7.
hCD300c-Ig ameliorates GVHD in mice. Lethally irradiated BALB/c recipients were injected i.v. with 5 × 106 BM and 2.5 × 106 spleen cells from C57BL/6 mice at day 0 and i.p. with 20 μg hCD300c-Ig, or control Ig every 3 days for 6 times. (A–C) Recipients were monitored for (A) survival, (B) weight change, and (C) clinical GVHD. (D,E) In separate experiments, recipients given 20 μg hCD300c-Ig or control Ig at 3-day intervals from days 0 to 12 were euthanized 2 weeks after BMT. (D,E) The liver, SI and lung were analyzed for histologic damage. (D) Representative photomicrographs (the magnification was X200), and (E) mean ± SD of histopathology scores. (F–M) hCD300c-Ig inhibits T-cell proliferation and activation in response to alloantigens in vivo. Lethally irradiated BALB/c mice were injected i.v. with 5 × 106 BM 10 × 106 splenic cells from C57BL/6 mice. The recipients were injected i.v. on day 0 and i.p. on day 2 with 20 μg hCD300c-Ig, or control Ig. On Day 4 post-transplant, the percentage of (F–I) Ki67+, (J,K) CD44hi, and (L,M) CD62Llo cells in donor T cells (H2b+CD4+, or H2b+CD8+) of the spleens were examined by flow cytometry. (F,H,J,L) Representative flow cytometric profiles and (G,I,K,M) statistical data are shown. (J,L) Dash lines: control Ig; solid lines: hCD300c. Pooled data from 2 separate experiments are represented; with 5–6 mice per group in each experiment. *P < 0.05 compared with control Ig-treated mice.