Abstract
Background:
Human papillomavirus (HPV) subtypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 have been implicated in the development of cervical cancer (CC). These 13 high risk HPV types have been shown to be present in up to 99.7% of CC samples. In Mexico, this cancer is the leading cause of death from malignancy among women. The aim of this study was to determine the prevalence of different HPV genotypes and investigate epidemiological aspects associated with HPV infection in women from Cozumel.
Material and methods:
We performed an epidemiological, prospective and cross sectional study with 1,187 who accepted participation in a campaign of screening for CC, during the period 2014 to 2015. Data on epidemiological and socio-economic variables were obtained. Cervical cells were collected for detection of HPV DNA and typing of HPV-positive samples by Multiplex PCR, using a commercial kit for 16 viral genotypes.
Results:
The overall prevalence of HPV in women from Cozumel was 15.8 % (188/1,187), either single (13.6%) or multiple (2.19 %). The most common HPV types , in descending order of frequency, were 58 (24.5 %), 59 (13.3 %), 39 (12.2 %) and 66 (9.6 %). The most frequent high risk types were HPV-58 and -59 and of low risk HPV types the most common was HPV-6. Number of sexual partners (OR=4.78; 95% CI= 2.73-8.37; P=<0.0001) and age of first coitus (OR=0.51; 95% CI=0.32-0.81; P=<0.0011) were significantly associated with HPV infection.
Conclusions:
Our data indicate that the overall incidence of high risk HPV infection in Cozumel is low as compared to other studies worldwide, with a different profile of subtypes. However, as expected, risky sexual behavior was found associated with positive cases of HPV. These results highlight the need for establish strategies to prevent HPV acquisition and evaluate the impact of a vaccine application in the Cozumel population.
Keywords: Human papillomavirus, HPV prevalence, HPV genotypes, cervical cancer
Introduction
Cervical cancer (CC) constitutes an important public health problem in the world, is the second most prevalent female cancer worldwide with 493,000 new cases occurring every year and 80% occurring in developing countries (Muñoz et al., 2003; Parkin and Bray, 2006).
In Mexico, this cancer is the leading cause of death from malignancy among women aged 25-64 years. In 2008, the national average mortality rate was 9.8 per 100,000 women aged 0-75 years, which corresponds to 9,800 deaths, 84% occurred in women with primary education or less and predominantly in women of reproductive age (Jemal et al., 2011).
Currently, it is known that the most important risk factor for the development of CC is genital infection with Human Papilloma Virus (HPV) (Walboomers et al., 1999; Bosch et al., 2002).
HPV infection in Mexico is one of the most common, among sexually transmitted infections. HPV infection is found in over 90% of cases of cervical cancer, but not all cases will develop cancer, this suggests that there are factors that are associated directly or indirectly with the increase in the likelihood of developing cancer or precursor lesions. These factors include smoking, nutritional status, the immune system, immunosuppression (particularly in the case of HIV infection) and other sexually transmitted infections. On the other hand, factors associated with the risk of acquiring HPV infection are age of onset of sexual activity, number of sexual partners, and history of sexually transmitted infections (Hildesheim et al., 2001).
HPV is a double-stranded, nonenveloped DNA virus of approximately 8000 base pairs. The HPV genome encodes 8 viral proteins that regulate the viral life cycle, of which it is known that the E5, E6 and E7 proteins are encoded by oncogenes that promote uncontrolled cell proliferation, inducing genomic instability, leading to initiation and progression of cancer (Scheffner et al., 1990; Hebner and Laimins, 2006).
More than 100 HPV genotypes are known, of which at least 40 types infect the human anogenital tract (de Villiers et al., 2004). The HPV can be cataloged into two types: low-risk and high-risk, according to their ability to cause malignant transformation and cancer develop. The low-risk HPV types causes benign hyperproliferative lesions or genital warts (condyloma acuminata), with a very limited tendency for malignant progression, while the high-risk HPV type is strongly associated with premalignant and malignant cervical lesions. The high-risk types, include HPV-16, -18, -31, -33, -35, -39, -45, -51, -52, -56, -58, -59, -66 and -68 and the low-risk types include HPV-6,-11 and -42, -44, causing warts on the cervix, vagina, vulva and anus in women and the penis, scrotum or anus in men (Muñoz et al., 2003).
The identification of high-risk human papillomavirus types as a necessary cause of cervical cancer offers the prospect of effective primary prevention and the possibility of improving the efficiency of screening for cervical cancer. In Mexico have made limited screening programs for early prevention of cervical cancer, in addition to having little information on the epidemiological factors associated with the presence of high-risk HPV. Therefore, the present study determines the prevalence of human papillomavirus genotypes, exploring their correlation with the sociodemographic, behavior and sexual factors in women from Cozumel-Quintana Roo, Mexico for estimating the impact of vaccines on CC and development of screening programs.
Materials and Methods
Study Subjects and selection criteria
An epidemiological, prospective and cross sectional study was conducted in 1187 women who accepted to participate in a campaign of screening for HPV in Cozumel, México, during the period December 2014 to June 2015. The study included women aged 20 to 70 years old apparently healthy independently of education levels, number of pregnancies, drug and alcohol consumption, hormone intake, or infection with other pathogens with known diagnosis. Epidemiological information (socio-demographic, sexual behavior and gyneco obstetric) was obtained from a standardized individual questionnaire.
Ethical aspects
All participants will provide written informed consent prior to the performance of any study-specific procedures. We will ensure protection of data privacy and respect of medically sensitive data of study participants.
Collection of specimens
Samples were collected from all women for HPV DNA test. Clinical samples were collected by vaginal scraping and scraping the exocervix with a cervical brush and stored in Universal Viral Transport System (UTM™Copan Diagnostics, USA) at room temperature until analysis.
DNA extraction.
For DNA extraction the Instangene Matrix (Bio-Rad, USA) was used according to the manufacturer’s instructions. Initially 200 µl of the samples was transferred to 0.6 mL tubes. Subsequently it was centrifuged at maximum speed for 5 minutes and the supernatant was removed. The pellet was washed 2 times with PBS 1X. Subsequently 50 µl of InstanGene Matrix was added to the pellet each sample. Purified DNA was diluted suspended in 50 µl of nucleases free water and was stored at -20 ° C.
PCR assays
Detection of 16 specific HPV types by multiplex fluorescent PCR was carried out using fHPV typing™ kit (Genomed, USA). The kit used 16 primers amplifying within E6 and E7 regions of the HPV genome of HPV types 6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68. PCR reactions were performed according to the manufacturer’s instructions. Amplification reactions were performed in a final volume of 5 μl containing 4 μl of reaction mix and 1 μl of DNA. PCR reactions were run as follow: 10 min at 95°C, 40 cycles of 95°C for 30 sec, 64°C for 30 sec and 72°C for 30 sec; final extension for 10 min at 60°C, in a thermal cycler (Applied Biosystems, Foster City, CA).
Capillary electrophoresis
PCR products were separated by size and color on an automated DNA analyzer ABI 3500 (Applied Biosystems). Electrophoresis reactions were performed in a final volume of 5.5 μl containing 4.5 μl of reaction mix (GeneScan 500 LIZ Size Standard and Hi-Di Formamide) and 1 μl of PCR product. The analysis of HPVf electrophoresis was performed using the program GeneMapper ID - X v1.3 (Applied Biosystems). HPV f-PCR products were detected on the electropherogram as peaks with a specific color and size. A human polymorphic DNA sequence (the STR d18S391) was amplified as internal control; the presence of this product reflects DNA integrity.
Results Interpretation
HPV Positive: The presence of HPV was detected as fluorescent peaks in the electropherogram. The Type was assessed by size and color of the peak. HPV Negative: the absence of HPV specific peaks and the presence of the internal control were characteristic of negative samples for all tested HPV types.
Statistical analysis
Percentages and frequencies of clinical data were calculated. Contingency HPV tables and its relationship to epidemiological variables were analyzed using chi-square and Fisher’s exact test, with confidence intervals of 95%. Data were analyzed with GraphPad Prism 5.0 (GraphPad Software, California). A p-value <0.05 was considered significant.
Results
HPV prevalence and genotype distribution
1,187 apparently healthy women aged 20–65 years from Cozumel were evaluated in this study. The overall prevalence of HPV was 15.83% (188/1187). A total of 15 viral types were identified; 175 (14.7 %) of women with High Risk HPV genotypes (HR-HPV) (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) and 13 women (1.09 %) with low risk HPV genotypes (LR-HPV) (6, 11). The most frequent in decreasing order were 58 (n = 46; 24.47 %), 59 (n = 25; 13.30 %) and 39 (n = 23; 12.23 %). Other HR-HPV types found in this study were 66, 16, 52, 18, 51, 31, 33, 56, 68 and 35. LR-HPV types most frequents was 6 (n =12). Of these positive samples 162 (13.64 %) had a single type and 26 (2.19 %) had multiple HPV type infections. The multiple infections have 2 or more combinations of HR-HPV. No combinations of LR-HPV were found. The most frequent associations of HR-HPV were HPV 51/59 (n=3; 11.53 %), followed by 58/59 (n=2; 7.69 %), and 16/68 (n=2; 7.69 %) associations.
Sociodemographic characteristics
Patients were stratified into groups according to age and presence of HR/LR-HPV infection. The average age was 45.50 years (range 40-49 years; n = 484; 40.78 %) of these 42.02% were positive for HPV genotypes (Figure 2), followed by women aged 50 to 59 years (n = 49; 26.06 %). When we analyzed the presence of HR-HPV and LR-HPV separately we observed that HR-HPV are observed in women with the highest prevalence from 40 to 49 years and decreasing with age, whereas LR-HPV had a peak highest prevalence in women aged 40-49 years (Figure 2). 42.29 % of the group had mostly Middle school education. No statistically significant differences between demographic characteristics (Table 1).
Figure 1.
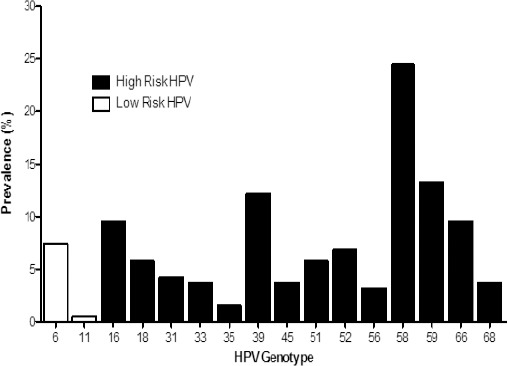
HPV Genotypes Prevalence Results. 15.83% of Positive cases had VPH infection, 14.7% of cases are positive for high-risk HPV and 1.09% is positive for low-risk HPV.
Figure 2.
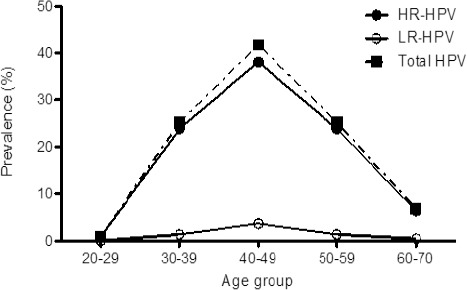
Age-specific HPV Genotypes Prevalence. 40.57% are Women with 40-49 Years.
Table 1.
Analysis of Demographic Factors according to HPV Status
| Demographic Variables | Overall (n=1187) | With HPV (n=188) | Without HPV (n=999) | OR | CI 95% | p-Value*+ |
|---|---|---|---|---|---|---|
| Age group (years) | 0.8445* | |||||
| 20-29 | 21 (1.77) | 2 (1.06) | 19 (1.90) | 1 | - | |
| 30-39 | 284 (23.93) | 45 (23.94) | 239 (23.92) | 1.7887 | 0.4025 - 7.9482 | |
| 40-49 | 484 (40.78) | 79 (42.02) | 405 (40.54) | 1.8531 | 0.4232 - 8.1150 | |
| 50-59 | 320 (26.96) | 49 (26.06) | 271 (27.13) | 1.7177 | 0.3877 - 7.6101 | |
| 60-70 | 78 (6.57) | 13 (6.91) | 65 (6.51) | 1.9000 | 0.3937 - 9.1696 | |
| Level of education | 0.8512* | |||||
| Uneducated | 306 (25.78) | 2 (1.06) | 10 (1.00) | 1 | - | |
| Elementary school | 502 (42.29) | 55 (29.26) | 251 (25.13) | 1.0956 | 0.2335 - 5.1414 | |
| Middle school | 256 (21.57) | 67 (35.64) | 435 (43.54) | 0.7701 | 0.1651 - 3.5917 | |
| High school | 110 (9.27) | 48 (25.53) | 208 (20.82) | 1.1538 | 0.2448 - 5.4380 | |
| College | 1 (0.08) | 16 (8.51) | 94 (9.41) | 0.8511 | 0.1704 - 4.2496 | |
| Postgraduate | 12 (1.01) | 0 (0.00) | 1 (0.10) | 1.4000 | 0.0429 - 45.681 | |
x2 test was used to compare between participants; + p-value <0.05 was considered significant.
The mean age of women with multiple infections was 43 years, but no association with any age group was found (P=<0.05 was considered significant).
Risk factors associated with infection of HPV types
The 58.89% of the group started their sex life on the range of age 15-20 years old and had mostly one or two sexual partners (73.8 %). An association between sexual behavior and HPV was found, number of sexual partners (OR=4.78; 95% CI= 2.73-8.37; P=<0.0001) and age of first coitus (OR=0.51; 95% CI=0.32-0.81) was significantly associated with HPV genotype infection (Table 2).
Table 2.
Analysis of Behavioral and Clinical Factors according to HPV Status
| Sexual behavior and clinical variables | Overall (n=1,187) | With HPV (n=188) | Without HPV (n=999) | OR | CI 95% | p-Value+,* |
|---|---|---|---|---|---|---|
| Age of first coitus (years) | 0.0011* | |||||
| <15 | 118 (9.94) | 31 (16.49) | 87 (8.71) | 1 | - | |
| 15-20 | 699 (58.89) | 109 (57.98) | 590 (59.06) | 0.5185 | 0.3279 - 0.8197 | |
| 20-25 | 292 (24.60) | 45 (23.94) | 247 (24.72) | 0.5113 | 0.3044 - 0.8589 | |
| 25-30 | 66 (5.56) | 3 (1.60) | 63 (6.31) | 0.1336 | 0.0391 - 0.4566 | |
| >30 | 12 (1.01) | 0 (0.00) | 12 (1.20) | 0.1111 | 0.0064 - 1.9323 | |
| Number of sexual partners | <0.0001* | |||||
| 1 | 549 (46.25) | 56 (29.79) | 493 (49.35) | 1 | - | |
| 2 | 327 (27.55) | 46 (24.47) | 281 (28.13) | 1.4412 | 0.9501 - 2.1860 | |
| 3 | 157 (13.23) | 37 (19.68) | 120 (12.01) | 2.7144 | 1.7124 - 4.3028 | |
| 4 | 71 (5.98) | 25 (13.30) | 46 (4.60) | 4.7845 | 2.7331 - 8.3760 | |
| >5 | 79 (6.66) | 24 (12.77) | 55 (5.51) | 3.8416 | 2.2088 - 6.6814 | |
| Previous sexually transmitted diseases | 0.1802* | |||||
| No | 1182 (99.58) | 186 (98.94) | 996 (99.70) | 1 | - | |
| Yes | 5 (0.42) | 2 (1.06) | 3 (0.30) | 3.5699 | 0.5924 - 21.511 | |
| Duration (in a year) since the last PAP | 0.0010* | |||||
| 1 | 324 (27.30) | 54 (28.72) | 270 (27.03) | 1 | - | |
| 2 | 244 (20.56) | 48 (25.53) | 196 (19.62) | 1.2245 | 0.7964 - 1.8826 | |
| 3 | 113 (9.52) | 25 (13.30) | 88 (8.81) | 1.4205 | 0.8347 - 2.4173 | |
| >4 | 192 (16.18) | 39 (20.74) | 153 (15.32) | 1.2745 | 0.8069 - 2.0132 | |
| Never has been done | 313 (26.37) | 22 (11.17) | 292 (29.23) | 0.3767 | 0.2234 - 0.6353 | |
| PAP test result | <0.0001* | |||||
| Normal | 840 (70.77) | 161 (85.64) | 679 (67.97) | 1 | - | |
| Abnormal | 3 (0.25) | 1 (0.53) | 2 (0.20) | 2.1087 | 0.1900 - 23.399 | |
| Never has been done | 344 (28.98) | 26 (13.83) | 318 (31.83) | - | - | |
| Duration (in a year) since the last colposcopy | 0.0148* | |||||
| 1 | 76 (6.40) | 16 (8.51) | 60 (6.01) | 1 | - | |
| 2 | 65 (5.48) | 16 (8.51) | 49 (4.90) | 1.2245 | 0.5562 - 2.6955 | |
| 3 | 46 (3.88) | 12 (6.38) | 34 (3.40) | 1.3235 | 0.5608 - 3.1234 | |
| >4 | 93 (7.83) | 18 (9.57) | 75 (7.51) | 0.9000 | 0.4234 - 1.9133 | |
| Never has been done | 907 (76.41) | 126 (67.02) | 781 (78.18) | 0.6050 | 0.3378 - 1.0835 | |
| Colposcopy result | <0.0001* | |||||
| Normal | 36 (3.03) | 38 (20.21) | 195 (19.52) | 1 | - | |
| Abnormal | 233 (19.63) | 17 (9.04) | 19 (1.90) | 4.5914 | 2.1886 - 9.6322 | |
| Never has been done | 918 (77.34) | 133 (70.74) | 785 (78.58) | - | - | |
Xi2test was used to compare between participants; °Fisher’s exact test was used to compare between participants; + p-value <0.05 was considered significant.
Previous sexually transmitted diseases were not found to be an important risk factor in this population, because most of the women surveyed reported not had having previous sexual disease transmission (99.58%).
The date of the last PAP showed a significant difference (p=0.0010) between HPV detection by PCR, as well as PAP test result with p<0.0001 showing concordance between cytology abnormalities by PAP and HPV presence (Table 2). The date last study of colposcopy also showed a difference between HPV groups with p=0.0148.
Discussion
The role of HPV in the development of cervical cancer has acquired a fundamental importance in recent years. In Mexico there are few studies to characterize genotypes associated with development of cervical cancer. This has allowed incorporation of new healthy systems and cervical cancer prevention strategies. These strategies include HPV screening studies through the detection of specific viral DNA; allows us to characterize the variability frequencies of specific HPV in a particular population. The authors have reported that the variation in the relative distribution of HPV genotypes may be determined by ethnic-geographic factors. A meta-analysis on the prevalence of HPV in Mexico published by Peralta-Rodríguez (2012) found that the most common types of HPV were 16 and 18 (71.7 % of 12 reports) (Peralta-Rodríguez et al., 2012).
These data are consistent with the information reported worldwide. However, when a regional analysis (central, southern and western of Mexico) is performed, significant differences are found in the prevalence of these populations. It has been determined that in central and southern regions of Mexico, the most common HPV found are -16/18 types. However, in western region, the fraction of HPV -16/58 is the most common, with a percentage of 90.3 % (Salcedo et al., 2014).
The present study reports the first screening and genotyping of HPV in the municipality of Cozumel (an island from east of Mexico). Our data show a prevalence of 15.83 % in HPV detection (188/1187) in healthy women with normal cytology study belonging to the municipality of Cozumel. This result corresponds to a relatively low prevalence; however, studies performed worldwide in women with normal cytology generally show a low prevalence of HPV subtypes. A study performed by Clifford (2005) with 15,613 samples of women from different countries reported a prevalence ranging between 1.4 % and 25.6 % (Clifford et al., 2005). In Mexico, cytology studies with normal results show prevalence between 3 to 14% (Lazcano-Ponce et al., 2001; Gonzalez et al., 2006; Sanchez-Anguiano et al., 2006).
We found that the most common HPV types are 58 and 59 (37.77 %) in women from Cozumel. In global terms, women with cervical injury have a higher prevalence of HPV genotype -58 (8.8%) (Liaw et al., 1997; Matsukura and Sugase, 2004). Another study published by Salcedo et al. report a prevalence of 5.4 % in -59 HPV type in normal cervical tissue, and 1% in samples of cervical cancer (Salcedo et al., 2014). These results suggest that these virus types have low adaptive capacity in neoplastic cells. It is therefore important to note that the study population corresponds to most women with apparently healthy and normal cytology results.
In our study, another high risk HPV types, of which HPV 58, 59, 39, 66, 16, 52, 18 and 51 collectively represent 14.7 % of the prevalence, were also detected. However, we obtained a low prevalence of genotypes 16 (n= 18, 9.57%) and 18 (n= 11, 5.85%), unlike worldwide reports. Although HPV 16 and 18 are the most frequent HPV genotypes, their relative importance varies worldwide (Smith et al., 2007).
In our study the prevalence of high risk HPV DNA was much higher in women between 40-49 years (40.78 %). Zhao et al., (2012) conducted a longitudinal study where samples of 26558 women with negative cytology were analyzed to compare high risk HPV presence. The prevalence of high-risk HPV in this study was higher in women under 30 years (8.1%) than in women aged between 40-49 years (2.8%). However, in women over 40 years, high-risk HPV did not decrease significantly in prevalence, unlike younger women. In our study, the average age range associated with high-risk genotypes was 45 years, and the HPV prevalence was declining at older ages. The decrease in the prevalence of HPV in aged women could be related by the sex activity or the immune response status of the women studied.
In this study we found an important correlation between sexual behavior and the presence of high-risk HPV genotypes. In case of onset of sexual life, significant differences between ages < 15 and 15-20 years (OR=0.51; 95% CI=0.32-0.81; P=<0.0011) were found. This is consistent with the results found in other reports, which indicate an increased risk of HPV infection in those who initiated sexual activity at an early age. Rombaldi et al., (2006) reported increased risk of HR-HPV in those who started their active sexual life before 17 years (OR = 1.6; 95% CI 0.8-3.0).
According to the number of sexual partners, most of the studied population had one or two sexual partners, however, participants who reported more than two sexual partners in their lifetime, showed an increased risk of HPV infection (95%; p = <0.0001). These findings are consistent with other studies, Kjellberg et al., (2000) report a 5 times higher risk in those who reported two or more sexual partners in their lifetime. Hangensee et al., (1999) also noted an association between the number of sexual partners and HPV infection in pregnant women (2 -5 sexual partners RM = 1.6; 95% CI=1.3-2.1). Chang-Claude et al., (1996) also reported an increased risk of HPV infection in those who reported more than three sexual partners (OR = 2.2; 95% CI=0.9-5.5).
The previous sexually transmitted diseases were also evaluated in this study. We found that 1.06 % (n = 2) of the HPV-positive population had a sexually transmitted disease. However we did not find significant differences between the variables previously described.
Sociocultural and demographic are very important parameters to understand the level of exposure of a population for a disease. The educational level study of the evaluated population can be considered as basic, so this could be a risk area for infectious diseases transmission and however, no significant differences between these variables were found.
On the other hand, it is well known that infection with high-risk genotypes has a high probability to develop a malignancy that lead in a cervical cancer. An appropriate follow up of the treatment for these women is needed in order to avoid the progression of the disease. The development of cervical cancer does not depend in the sensitivity of the DNA test or the ability to detect high-risk genotypes, but the constant repetition of the same and the systematic monitoring of women with positive results.
Finally, our data analysis can be considered in order to evaluate the impact of vaccine introduction and future cancer prevention strategies. Therefore, according to our results a customized HPV vaccine could be required for the prevention and treatment of cervical lesions in women from Cozumel or east of Mexico.
Funding Statement
This work was supported by Ayuntamiento Constitucional de Cozumel grants bidding IA-823001999-N27-2014.
Acknowledgments
The authors thank of the Laboratory Central ADN SA de CV, agreed to participate and made possible the execution of this study. This work was also supported by Yendi Basto Cruz and Emily Aranda López.
References
- Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Claude J, Schneider A, Smith M, et al. Longitudinal study of the effects of pregnancy and other factors on detection of HPV. Gynecol Oncol. 1996;60:355–62. doi: 10.1006/gyno.1996.0055. [DOI] [PubMed] [Google Scholar]
- Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys:a pooled analysis. Lancet. 2005;366:991–8. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Losa MR, Hidalgo-Martínez AC, Manzano-Cabrera L, Estrada-Saldivar A, Puerto-Solis M. Absence of DNA human papillomavirus type 16 in Mexican women with normal pap smear. Int J Virol. 2006;2:59–62. [Google Scholar]
- Hagensee ME, Slavinsky J, Gaffga CM, et al. Seroprevalence of human papillomavirus type 16 in pregnant women. Obstet Gynecol. 1999;94:653–8. doi: 10.1016/s0029-7844(99)00454-8. [DOI] [PubMed] [Google Scholar]
- Hebner CM, Laimins LA. Human papillomaviruses:basic mechanisms of pathogenesis and oncogenicity. Rev Med Virol. 2006;16:83–97. doi: 10.1002/rmv.488. [DOI] [PubMed] [Google Scholar]
- Hildesheim A, Herrero R, Castle PE, et al. HPV co-factors related to the development of cervical cancer:results from a population-based study in Costa Rica. Br J Cancer. 2001;84:1219–26. doi: 10.1054/bjoc.2001.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kjellberg L, Hallmans G, Ahren AM, et al. Smoking, diet, pregnancy and oral contraceptive use as risk factors for cervical intra-epithelial neoplasia in relation to human papillomavirus infection. Br J Cancer. 2000;82:1332–8. doi: 10.1054/bjoc.1999.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazcano-Ponce E, Herrero R, Muñoz N, et al. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91:412–20. doi: 10.1002/1097-0215(20010201)91:3<412::aid-ijc1071>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Liaw KL, Hsing AW, Schiffman MH, et al. Human papillomavirus types 52 and 58 are prevalent in cervical cancer from Chinese women. Int J Cancer. 1997;73:775–6. doi: 10.1002/(sici)1097-0215(19971127)73:5<775::aid-ijc27>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Matsukura T, Sugase M. Human papillomavirus genomes in squamous cell carcinomas of the uterine cervix. Virology. 2004;324:439–49. doi: 10.1016/j.virol.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Muñoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F. The burden of HPV-related cancers. Vaccine. 2006;24:11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- Peralta-Rodríguez R, Romero-Morelos P, Villegas-Ruíz V, et al. Prevalence of human papillomavirus in the cervical epithelium of Mexican women:meta-analysis. Infect Agent Cancer. 2012;7:1–34. doi: 10.1186/1750-9378-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo M, Pina-Sanchez P, Vallejo-Ruiz V, et al. Human papillomavirus genotypes among females in Mexico:a study from the Mexican institute for social security. Asian Pac J Cancer Prev. 2014;15:10061–6. doi: 10.7314/apjcp.2014.15.23.10061. [DOI] [PubMed] [Google Scholar]
- Sanchez-Anguiano LF, Alvarado-Esquivel C, Reyes-Romero MA, Carrera-Rodriguez M. Human papillomavirus infections in women seeking cervical Papanicolaou cytology of Durango, Mexico:prevalence and genotypes. BMC Infect Dis. 2006;6:27. doi: 10.1186/1471-2334-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions:a meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- Rombaldi RL, Serafini EP, Villa LL, et al. Infection with human papillomaviruses of sexual partners of women having cervical intraepithelial neoplasia. Braz J Med Biol Res. 2006;39:177–87. doi: 10.1590/s0100-879x2006000200003. [DOI] [PubMed] [Google Scholar]
- Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Zhao CQ, Yang HT, Xue DB, Yang M. High-risk human papillomavirus DNA testing and histologic follow up in women with abnormal cytology. Zhonghua Bing Li Xue Za Zhi. 2012;41:774–8. doi: 10.3760/cma.j.issn.0529-5807.2012.11.017. [DOI] [PubMed] [Google Scholar]


