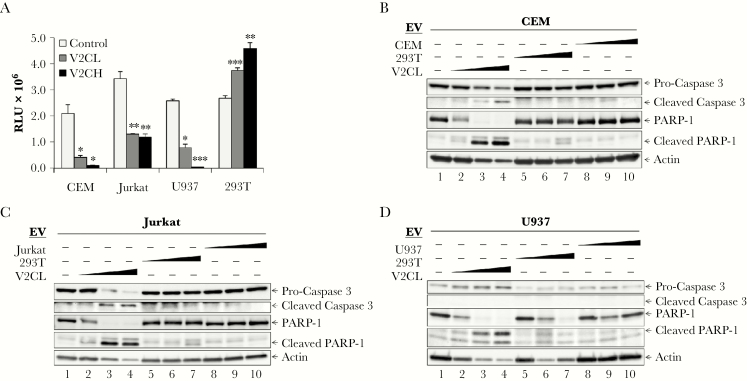
Figure 8.
Recipient monocyte and T-cell apoptosis. (A) Supernatants from 293T (Control), V2CL, and V2CH cells (5 days, 0.22 µm filtered, in exosome-free media) were used to treat CEM, Jurkat, U937, and 293T recipient cells. Recipient cells were seeded in a 96-well plate at 5 × 105 cells in 50 μL of fresh media, treated with 50 μL of filtered supernatants, and incubated 5 days, followed by analysis by CellTiter-Glo for cell viability. Statistical analysis by Student’s 2-tailed t test compares supernatant-treated groups with control cells of the same type (*, P < .05; **, P < .01; ***, P < .001). 293T, V2CL, CEM, Jurkat, and U937 cells were grown in exosome-free media for 5 days, followed by harvesting and filtering (0.22 µm) of the supernatant. Log-phase CEM, Jurkat, and U937 cells were plated at a density of 1 × 106 cells/mL and treated with increasing concentrations (100, 250, and 500 μL) of supernatant from either 293T, V2CL, or their own cell type. Cells were incubated for 5 days, followed by harvesting of the cells and lysis. Lysates of CEM (B), Jurkat (C), and U937 (D) cells were then run on a 4–20% Tris-glycine gel and subjected to western blot analysis for apoptotic markers procaspase 3 and PARP-1 and their cleaved forms, and Actin.