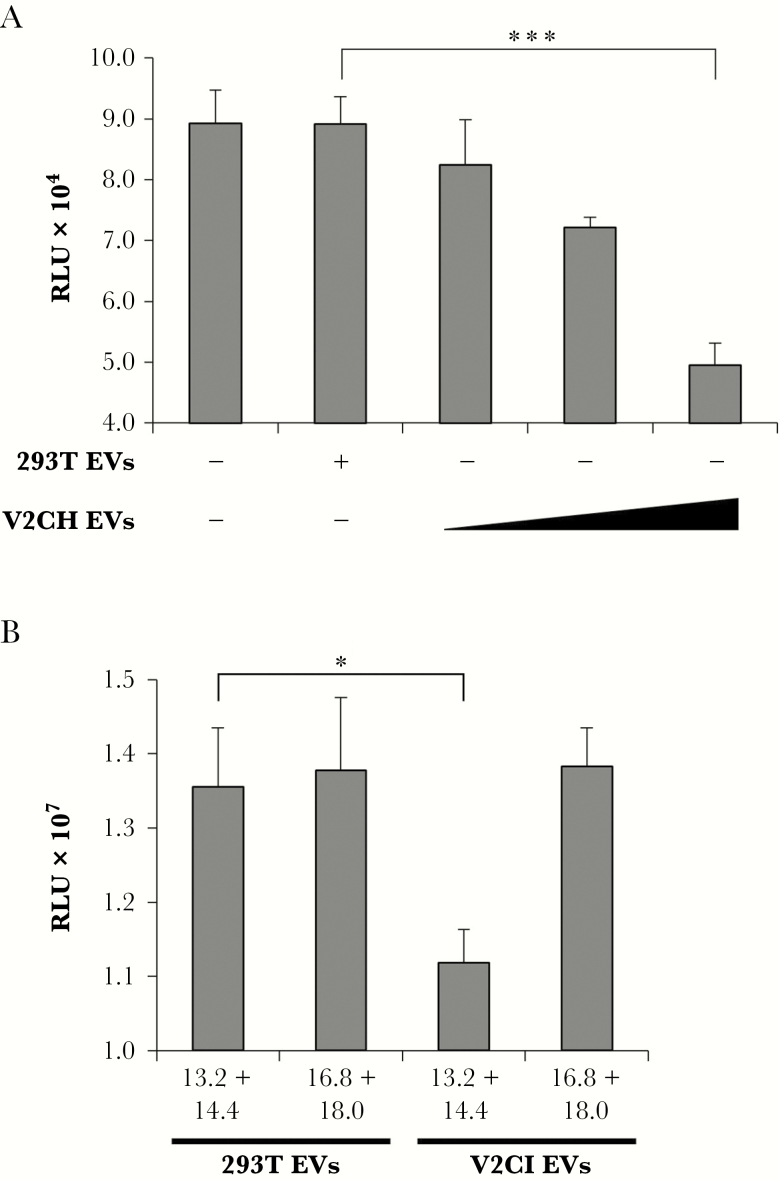
Figure 9.
Induction of recipient T-cell death by purified VP40 EVs. (A) 293T and V2CH cells were grown in exosome-free media for 5 days, followed by harvesting of cell-free supernatants and filtration through 0.22 μm. Supernatants were then spun at 100000 ×g for 90 minutes to pellet EVs, followed by resuspension in sterile 1× PBS. Concentrations of resulting ultracentrifuged EVs were determined with ZetaView analysis, followed by treatment of CEM cells with increasing concentrations of EVs (10000, 25000, or 75000 particles/cell) from V2CH cell type. Controls included CEM cells that were left untreated and CEM cells that received a treatment of the highest concentration of 293T cells (75000 particles/cell). Cells were incubated for 3 days followed by analysis of cell viability by CellTiter-Glo. (B) 293T and V2CI cells were grown in exosome-free media for 5 days. Cell-free supernatants were harvested and incubated with equal volumes of ExoMAX overnight at 4°C. The EVs were pelleted, resuspended in 400 µL sterile 1× PBS, and loaded onto a 6–18% iodixanol density gradient (1.2% increments). Samples were ultracentrifuged for 90 minutes at 100000 ×g, followed by harvesting and isolation of each fraction. Select fractions (13.2 + 14.4 and 16.8 + 18.0) were pooled and subjected to a second ultracentrifuge spin for 90 minutes at 100000 ×g diluted in 1× PBS to pellet the EVs away from residual iodixanol. Resulting EV pellets were resuspended in 100 µL sterile 1× PBS and used to treat recipient CEM cells at a concentration of 10000 particles per cell (concentrations determined by ZetaView analysis). Cells were incubated for 5 days followed by analysis of viability by CellTiter-Glo assay. Statistical analysis by Student’s 2-tailed t test compares groups treated with EVs from V2C cells to those treated with EVs from 293T cells (*, P < .05; ***, P < .001).