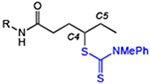
Table 1.
Site-specific C–H dithiocarbamylation of simple substrates.[a]
| entry | product | blue LEDs yield (%)[b] | radical initiator yield (%)[c] |
|---|---|---|---|
 |
|||
| 1 | 1: R = iPr | 91 | 73 |
| 2 | 2: R = tBu | 61 | 56 |
| 3 | 3: R = nPr | 79 (3:1 C4:C5) | 85 (3:1 C4:C5) |
| 4 | 4: R = nHex | 87 (2.5:1 rr)[d] | 87 (1.6:1 rr)[d] |
 |
|||
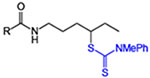
| 5 | 5: R = Ph | 82 | 82 |
| 6 | 6: R = CF3 | 78 | 86 |
| 7 | 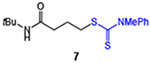 |
65 | 76 |
| 8 | 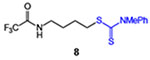 |
21 (35% brsm) | 52 |
| 9 | 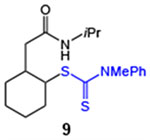 |
89 (2:1 dr) | 79 (2:1 dr) |
| 10 | 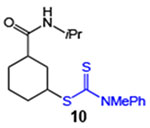 |
81 (2:1 dr) | 87 (2:1 dr) |
Isolated yields.
Reactions were performed with blue LEDs in PhCF3 under ambient conditions for 14 h under Ar
Reactions were performed in PhCl at 120 °C with 10 mol % dicumyl peroxide for 14 h under Ar
Regioisomeric mixture resulting from 1,5-functionalization of the amide sidechain.
