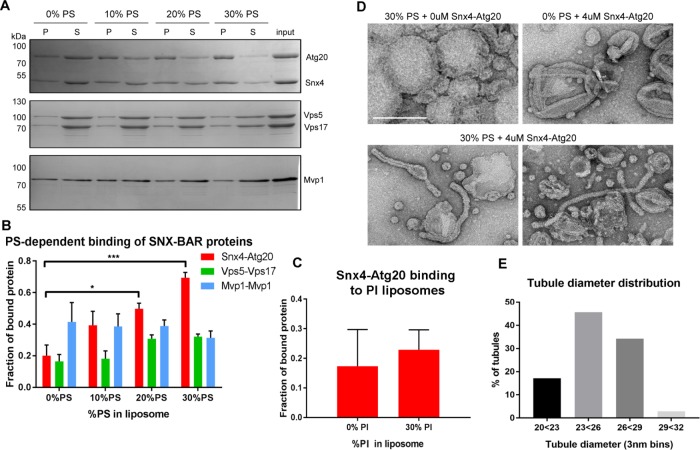
FIGURE 5:
The Snx4-Atg20 heterodimer exhibits membrane remodeling activity. (A) Representative Coomassie stained gels of liposome sedimentation reactions containing SNX-BAR complexes and liposomes with indicated amounts of PS. The position of mass standards (kDa) are indicated. (B) Quantification of Snx4-Atg20 binding to liposomes. Fraction of bound protein is calculated as a fraction of SNX-BAR proteins in the pellet fraction (P) over the sum of pellet and supernatant (S) fraction and is averaged over at least three independent experiments. *p ≤ 0.05; ***p ≤ 0.001. (C) Quantification of Snx4-Atg20 binding to liposomes containing 0% or 30% PI. Fraction of bound protein is calculated as a fraction of SNX-BAR proteins in the pellet fraction (P) over the sum of pellet and supernatant (S) fraction and is averaged over at least three independent experiments. The difference is not statistically significant. (D) A gallery of micrographs of negative stained liposomes with (30%) or without PS (0%) incubated with (4 μM) or without (0 μM) Snx4-Atg20 heterodimer is shown. The scale bar represents 200 nm. (E) Histogram of the diameters of tubules generated by 4 μM Snx4-Atg20 heterodimer (n = 35) in 3 nm bins is shown. Snx4-Atg20 generated average tubule diameters of 25.2 ± 2.1 nm.