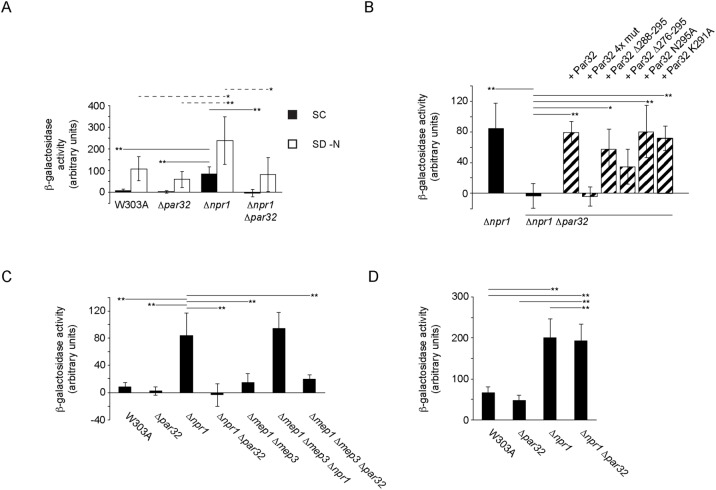
FIGURE 7:
Par32 and Npr1 regulate expression of Gap1 in SC-grown cells. (A) Strains, as indicated, expressing the Gap1-lacZ reporter construct were grown in SC or under nitrogen starvation (SD–N, 5 h) conditions. Cells were then lysed, and β-galactosidase activity was determined. For cells grown in SC (filled boxes, mean ± s.d., n = 9–13 independent experiments), the mean β-galactosidase activity levels for the indicated strains were significantly heterogeneous (one-way ANOVA; F3,36 = 47.96, hence p = 1.17 × 10–12). Significant differences between pairs, as determined using the post-hoc Tukey HSD test, are indicated above the relevant pairs using full lines. Mean β-galactosidase activity levels for cells grown in SD–N (open boxes, n = 5–8 independent experiments) were also significantly heterogeneous (one-way ANOVA; F3,21 = 6.74, hence p = 0.0023). Significant differences between relevant pairs are indicated with dotted lines (*p < 0.05; **p < 0.01). (B) The four GRGGAG motifs in Par32 are required for Gap1 expression. Δnpr1Δpar32 cells expressing the Gap1-lacZ reporter construct and the indicated Par32 constructs were grown in SC and compared with W303A and Δnpr1 cells expressing the reporter. Cells were lysed, and β-galactosidase activity was determined. The mean β-galactosidase activity levels were significantly different (one-way ANOVA; F7,28 = 12.27, hence p = 4.18 × 10–7; n = 3–9 independent experiments). Significant differences between relevant pairs, as determined using the post-hoc Tukey HSD test, are indicated (*p < 0.05; **p < 0.01). (C) Regulation of Gap1 expression in ammonium-replete conditions (SC) by Npr1, and Par32 is independent of Mep1 and Mep3. The indicated strains expressing the Gap1-lacZ reporter construct were grown in SC and lysed prior to determination of β-galactosidase activity. The means of β-galactosidase activity levels for the various strains were significantly different (one-way ANOVA; F6,46 = 37.44, hence p = 4.44 × 10–16). Significant differences between selected pairs, as determined using the post-hoc Tukey HSD test, are indicated (**p < 0.01). (D) Par32-mediated repression of Gap1 expression is ammonium dependent. The experiment was performed as in C, but the cells were grown in SD–ammonium. The means of β-galactosidase activity levels for the various strains were significantly different (one-way ANOVA F3,8 = 19.36, hence p = 0.0005). Significant differences between selected pairs, as determined using the post-hoc Tukey HSD test, are indicated (**p < 0.01).