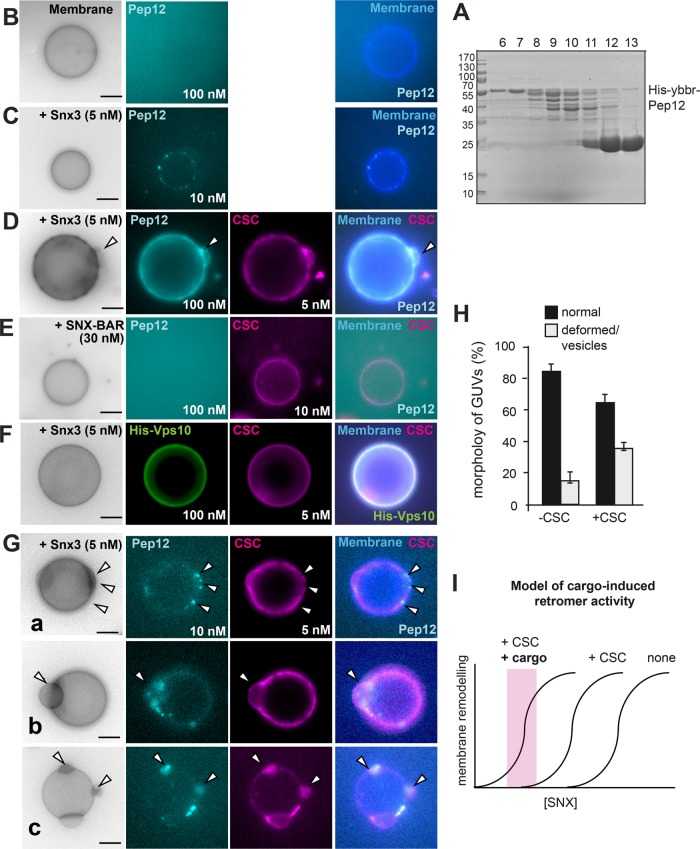
FIGURE 6:
Cargo-induced vesiculation and domain formation by the Snx3-retromer. (A–C) Purification and analysis of Snx3 cargo. Purification of the cytosolic domain of Pep12 (A). N-terminally His-tagged Pep12 (amino acids 1–266) was purified via Ni-NTA resin, the His-tag was removed by protease cleavage, and the eluate was applied to a gel-filtration column. Fractions were analyzed by SDS–PAGE and Coomassie staining (see Materials and Methods). Fractions 6 and 7 were pooled and labeled as described under Materials and Methods and added to GUVs in the absence (B) or presence (C) of non-GFP-tagged Snx3 (5 nM) for 15 min and analyzed by fluorescence microscopy. (D) Pep12 triggers Snx3-retromer membrane remodeling activity. Snx3 and CSC (5 nM each) were added with 100 nM Pep12 to GUVs and analyzed as before. (E) SNX-BAR retromer addition together with Pep12 to GUVs. SNX-BAR complex (30 nM) and CSC (10 nM) were added and analysis was done as in D. (F) Snx3-retromer does not cluster the Vps10 cargo. His-tagged Vps10 (100 nM) was added together with Snx3 (5 nM) and CSC (5 nM) to GUVs. (G) Early events of cargo-induced membrane deformation by Snx3-retromer. Snx3 and CSC (5 nM each) were added together with 10 nM Pep12 to the GUVs. Arrows in examples a–c show distinct events of membrane deformation and Pep12-CSC colocalization. (H) Quantification of membrane deformation events by Pep12 (10 nM) and Snx3 (5 nM) in the absence of presence of CSC (5 nM) as observed in C and G. Data are represented as mean ± SD (20–35 GUVs from three independent experiments were analyzed for quantification). Scale bar, 5 µm. (I) Model of cargo-induced retromer activity. Without CSC and cargo, more SNX is needed to remodel membrane. CSC and cargo would lower the needed amount of SNX protein on membrane. Note that the curves do not reflect actual measurements but are models based on the findings. For details see the text.