FIGURE 6:
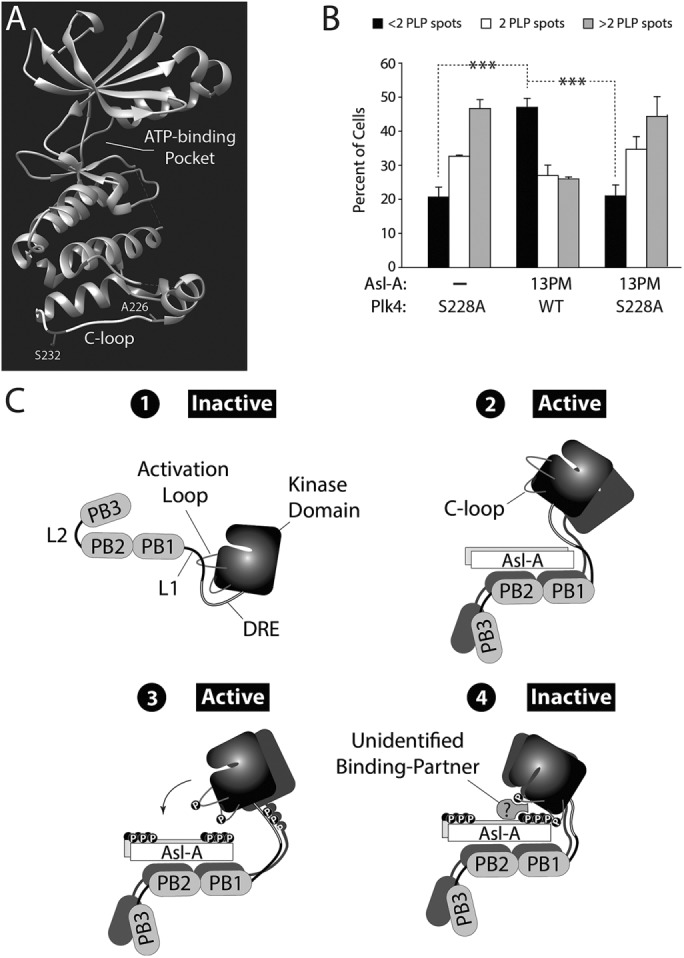
Plk4 phosphorylates its kinase domain and Asl-A, generating a state that inhibits kinase activity. (A) Atomic structure of the human Plk4 kinase domain (Wong et al., 2015). The residue, A226, is the human equivalent of Drosophila S228 within the C-loop. Human Plk4 autophosphorylates C-loop residue S232 in vitro (Sillibourne et al., 2010). (B) Asl-A-13PM does not inhibit Plk4-S228A from amplifying centrioles. Plk4-GFP/Asl-A-V5 dual-gene expression plasmids were transfected into cells and the next day were induced to express for 72 h. Centrioles were visualized with PLP immunostaining, and the number of centrioles per cell was counted. n = 3 experiments per construct (total 300 cells/construct). (C) Model: the phosphorylation state of Asl-A regulates Plk4 kinase activity by a multistep process of stimulation followed by negative feedback. 1, Initially, Plk4 is autoinhibited by its L1 domain, which masks its activation loop. 2, Nonphosphorylated Asl-A binds Plk4 and relieves autoinhibition to activate the kinase. 3, Homodimerized Plk4 phosphorylates itself (including its activation and C-loops) and, importantly, Asl-A. 4, Together, phospho-Asl-A and the phospho-C-loop recruit an unknown factor(s) to inactivate Plk4, perhaps by generating a complex that obstructs or distorts the active site.
