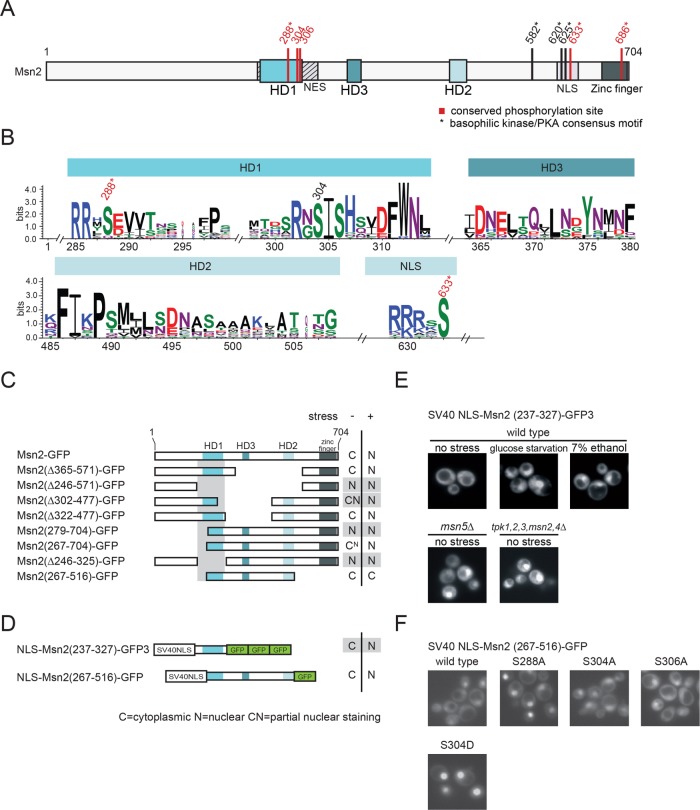
Figure 1:
Functional analysis of conserved regions of Msn2-related proteins. (A) Scheme of S. cerevisiae Msn2. Locations of the homology domains HD1 and HD2 and the newly identified HD3, as well as the NES, the NLS, and the zinc-finger DNA-binding domain are indicated. Conserved phosphorylation sites highlighted in red. * indicates a canonical PKA consensus motif (R/K-R/K-X-S/T). (B) Similarity plot of 19 Msn2 and 5 Msn4 homologues. Only results obtained for HD1, HD2, HD3 and a highly conserved phosphoaccepting residue (S633) of the NLS are shown. Conserved phosphorylation sites are highlighted. (C–F) The Msn2 region, comprising residues 237–327, constitutes a stress– and PKA signaling–regulated minimal NES. (C, D) Localization analysis of a series of Msn2-GFP truncations and derivatives. W303 msn2∆ msn4∆ cells carrying plasmids expressing Msn2-GFP derivatives were grown to logarithmic phase (–) and subsequently stressed with 7.5% ethanol (+). N: a nuclear GFP signal. C: cytoplasmic. CN and CN: partial nuclear staining. (E) Msn2 residues 237–327 were fused to the SV40 NLS and three tandem GFP units. A variety of physicochemical stresses (here 7% ethanol or glucose starvation) promote accumulation of SV40 NLS-Msn2(237-327)-GFP in the nucleus. Nucleocytoplasmic transport of the fusion protein also requires Msn5 and PKA signaling. (F) Effects of serine–to–alanine or –aspartic acid substitutions at conserved residues of the Msn2 NES.