Abstract
Background:
A definite cause and effect relationship between tobacco use and caries are lacking in literature.
Aim:
The aim of this study is to determine the influence of tobacco dependence on caries development in young male adults.
Materials and Methods:
Three hundred healthy adult males in the age group of 20–40 years were divided into three groups containing 100 participants each. Group A: participants using smokeless tobacco; Group B: participants who smoke tobacco; and Group C: participants who have never used tobacco. Their dependence on tobacco was assessed using the Fagerstrom test for nicotine dependence. Their dental caries status was assessed using decayed-missing-filled teeth/decayed-missing-filled surfaces (DMFT/DMFS) index adopted by the WHO (1987). Observations were statistically analyzed using Mann–Whitney test, and P < 0.05 was considered statistically significant.
Results:
Mean DMFT/DMFS was significantly higher for Groups A and B as compared to Group C. Within Group A, mean DMFT/DMFS increased significantly with increased dependence on smokeless tobacco. Within Group B, highest DMFT/DMFS was found in participants with low dependence on smoked tobacco while least mean DMFT/DMFS was found in participants with high dependence.
Conclusion:
Both forms of tobacco proved to be a significant risk factor for increased caries development. Thus, tobacco use should be an important factor in caries risk assessment of patients.
Keywords: Caries, nicotine dependence, smokeless tobacco, smoking, tobacco
INTRODUCTION
According to the national household survey of drug and alcohol abuse (2002), the overall prevalence of the current tobacco use was 55.8% in India.[1] In India, tobacco is consumed in various smokeless and smoked forms. Nicotine, found in tobacco, is highly addictive and responsible for tobacco dependence. Almost 30% of Indians older than age 15 years uses some form of tobacco.[2] Tobacco has a number of detrimental effects on oral health. It includes tooth loss, periodontal disease, oral soft-tissue changes, excessive wear of teeth, halitosis, staining, reduced taste sensation, implant failure, oropharyngeal cancer, and dental caries.[3] Cleft lips and palates are twice as common among children born to mothers who smoked during pregnancy.[4] The use of breath-freshening mints to alleviate the bad breath can cause dental erosion due to the large quantities of sugar and citric acid present in them.
Early literature reported that smoking helps to reduce dental caries.[5] It was stated that thiocyanate concentration, a constituent of tobacco smoke and normal saliva with possible caries-inhibiting effect, was found to be higher in smoker's saliva.[6] A case–control study comparing dental caries in smokeless tobacco users and nonusers also reported higher percentage of caries among nonusers.[7] However, recent studies have recognized tobacco use as a risk factor for coronal and root caries.[8,9,10,11] Holmén et al. did a longitudinal study in 10,068 adolescents, and yearly data on caries and tobacco use (cigarette smoking and use of smokeless tobacco) showed that tobacco use was clearly associated with increased caries increment.[10]
There is a general consensus that tobacco is associated with increased caries rate but this cause and effect relationship is not firmly proven. Therefore, the aim of this study was to assess the influence of smoked as well as smokeless tobacco on dental caries development in young adults. Furthermore, local and systemic effects of tobacco on oral cavity depend on various factors such as method, frequency, and duration of use and are dose dependent. Hence, this study also aimed to determine whether increased dependence on tobacco causes increased caries development.
MATERIALS AND METHODS
Three hundred healthy adult males, in the age group of 20–40 years, were included in this cross-sectional study. There were three study groups of 100 participants each.
Group A: participants using smokeless form of tobacco (four or more times in a day) for >2 years
Group B: participants using smoked form of tobacco (four or more times in a day) for >2 years
Group C (control group): participants who have never used any form of tobacco.
Patients over 40 years and < 20 years of age, female patients, patients taking drugs which alter salivary parameters, those using both smoked and smokeless tobacco, those who consumed alcohol, patients with any systemic disease, history of radiation therapy, salivary gland diseases, and patients who wore dentures were excluded from the study.
The aim and method of the study were explained to all the participants, and written consent was obtained. First, nicotine dependence of all the participants in Group A and B was assessed using the Fagerstrom test for nicotine dependence. The Fagerstrom test consists of six questions with their response options and corresponding score, 0 being the minimum and 10 being the maximum score. There are separate questionnaires for smokeless[12] and smoked[13] forms of tobacco. Scores of the questionnaire were added for each participant in Groups A and B to assess their nicotine dependence as follows: score 1–2 – low dependence; score 3–4 – low-to-moderate dependence; score 5–7 – moderate dependence; and score >8 – high dependence.
Next, dental caries status was assessed in all three groups using decayed-missing-filled (DMF) index adopted by the WHO (1987). DMF index which was introduced by Klein et al.[14] in 1938 and modified by the WHO[15] has two components as follows:
DMF teeth index (DMFT) measures the prevalence of dental caries
DMF surfaces index (DMFS) measures the severity of dental caries.
Intergroup and intragroup observations were statistically analyzed by Mann–Whitney test of significance using SPSS 16.0 Software (SPSS Inc., released 2007. SPSS for Windows, Version 16.0. Chicago, IL, USA), and P < 0.05 was considered statistically significant.
RESULTS
In Group A (smokeless tobacco), of the 100 participants assessed for nicotine dependence, 36 had low dependence, 24 had low-to-moderate dependence, 38 participants had moderate dependence, and only 2 had high nicotine dependence. In Group B (smoked tobacco), of the 100 participants assessed for nicotine dependence, 50 had low dependence, 28 had low-to-moderate dependence, 20 participants had moderate dependence, while just 2 had high nicotine dependence.
Intergroup comparisons
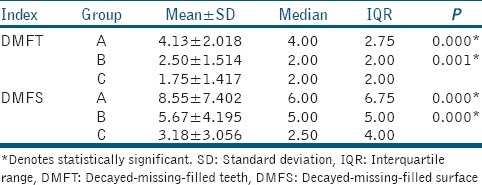
When DMFT index was compared for three groups, mean DMFT was found to be highest for Group A, followed by Group B and least in Group C (control group). The differences between Group A and B when compared to C (control group) were statistically significant. Similar results were obtained when mean DMFS was compared for three groups, i.e., highest mean DMFS for Group A, followed by B and least mean DMFS was found for Group C [Table 1].
Table 1.
Intergroup comparison of decayed-missing-filled teeth and decayed-missing-filled surface indices
Intragroup comparisons
For Group A (smokeless tobacco), DMFT/DMFS indices were compared for different levels of nicotine dependence in tobacco chewers. As the level of nicotine dependence increased from “low” to “low to moderate” to “moderate” among study participants, mean DMFT/DMFS also increased. All these differences were statistically significant (P < 0.05). From “moderate” to “high” dependence, DMFT and DMFS decreased; however, the difference was not statistically significant (P > 0.05) [Table 2].
Table 2.
Intragroup comparison of decayed-missing-filled teeth and decayed-missing-filled surface indices in Group A (smokeless tobacco) and Group B (smoked tobacco)
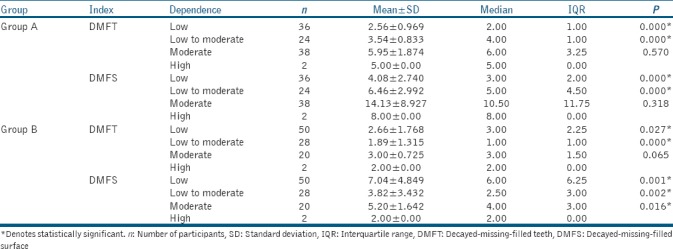
For Group B (smoked tobacco), mean DMFT index in patients with “low” nicotine dependence was 2.66 ± 1.768. In patients with “low-to-moderate” dependence, it decreased to 1.89 ± 1.315. In “moderate” dependence, mean DMFT again increased to 3.00 ± 0.725, which again decreased to 2.00 ± 0.00 in patients with “high” dependence. Similar results were found in DMFS index. Mean DMFS index in patients with low, low-to-moderate, moderate, and high dependence was 7.04 ± 4.849, 3.82 ± 3.432, 5.20 ± 1.642, and 2.00 ± 0.00, respectively [Table 2].
DISCUSSION
The results of this study clearly show that caries prevalence (as measured by DMFT index) and caries severity (as measured by DMFS index) are significantly higher in participants using tobacco as compared to nontobacco users (control group). This proves that tobacco has a definitive role to play in caries development. Our results are similar to those of other recent studies[8,9,10,11] and discard the older view of tobacco having a caries protective influence.[5,6,7] Furthermore, between Groups A and B, smokeless tobacco users had significantly higher DMFT/DMFS index than smoked tobacco users. This indicates that smokeless forms of tobacco are more detrimental than smoked forms of tobacco in caries development. It may be attributed to the fact that smokeless forms of tobacco have higher amounts of caries promoting factor such as sugars as compared to smoked forms of tobacco.
Nicotine is the cause of tobacco dependence. For smokeless tobacco, intragroup comparisons showed that with increasing nicotine dependence, caries prevalence and caries severity increased significantly in the study participants. The differences between “low” and “low-to-moderate” dependence as well as between “low-to-moderate” and “moderate” dependence were significant. It was found that DMFT/DMFS decreased slightly from “moderate” to “high” dependence although this was not a statistically significant difference. This proves that the effect of smokeless tobacco on caries is frequency and dose dependent. Greater risk of caries due to smokeless tobacco can be attributed to prolonged exposure to sugars present in smokeless forms of tobacco. Sugar content averages as much as 34% in different preparations of smokeless tobacco.[16] Sugars are added to have a neutralizing effect on the bitter taste of tobacco.[17] On an average, a wad of tobacco is kept in the oral cavity for 30 min, and hence, this prolonged duration of chewing tobacco also creates an environment conducive to dental caries. Nicotine changes the oral environment in other ways too. An in vitro study suggested that nicotine increases biofilm formation.[18] Smokeless tobacco extracts have shown to increase in vitro growth of Streptococcus mutans, Streptococcus sanguis, and Lactobacillus.[17] Effect of nicotine on salivary parameters also plays an important role in caries development. Excessive use of smokeless tobacco results in degenerative changes of more than 40% of minor salivary glands located at the site of chronic tobacco placement.[19] This causes reduction in saliva in oral cavity and promotes caries. Similar results were also stated by Kanwar et al. in their clinical study. They showed that long-term consumption of tobacco reduces salivary flow rate. Their study also showed that tobacco chewers have lower salivary pH than nonchewers probably because of lime present in smokeless tobacco. Lime reacts with bicarbonate buffering system turning saliva more acidic and conducive to caries development.[20]
Intragroup comparison in Group B (smoked tobacco) revealed results very much different from smokeless tobacco. Although DMFT/DMFS for Group B was statistically higher than the control group, DMFT/DMFS did not increase with increased dependence on smoked tobacco as seen in Group A. Between “low” and “low-to-moderate” dependence, DMFT/DMFS decreased significantly. Between “low-to-moderate” and “moderate” dependence, DMFT/DMFS again increased significantly. DMFT/DMFS again decreased from “moderate” to “high” dependence, but the difference was insignificant for DMFT and significant for DMFS index. Hence, no correlation between the degree of tobacco dependence and caries prevalence (DMFT) and severity (DMFS) was observed in tobacco smokers. In smokers, it was also observed that with higher dependence on smoked tobacco, gingival and periodontal problems increased. There were more missing teeth with increased nicotine dependence, but it was due to periodontal reasons and not caries, and hence, DMFT/DMFS did not increase. However, this observation was not recorded or analyzed. Similar to smokeless tobacco, sugars are also used as cigarette additives to serve as flavor/casing and humectants.[17] Tobacco smoke changes salivary parameters adding to caries promoting effect. Effect of nicotine on gustatory reflex appears to be initial stimulation followed by depression. Long-term use of tobacco decreases the sensitivity of taste receptors resulting in depressed salivary reflex. Consequentially, there is altered taste response and decreased salivary flow in tobacco users.[20,21] Salivary buffering capacity in smokers is also found to be approximately 20% lower than in nonsmokers leading to acidic pH.[20,21] Caries promoting microbiota also increase in saliva due to tobacco smoke. Lactobacilli colony count was found significantly higher in smokers as compared to nonsmokers in a clinical study.[22] Authors reported that this could be due to two reasons: first, more carious teeth in smokers as compared to nonsmokers and second, it was suggested that tobacco smoking depresses the immunoglobulins in oral cavity (IgM and IgA) leading to increase in bacteria.
There were some limitations to this study. The severity of observed periodontal disease was not documented. Therefore, a causal association between smoking and tooth loss could not be established. Second, caries is also influenced by confounding factors such as the socioeconomic status, oral hygiene, and malocclusion in participants. These factors were not taken into account in this study. Third, tobacco use was self-reported in this study which is not very reliable. Future studies using serum cotinine as a measure to assess nicotine levels in participants can be done. Finally, for the assessment of caries risk relation with nicotine dependence, the number of participants in different levels of dependence was not equally distributed. These shortcomings need to be overcome in the future research.
According to our literature search, even with these limitations, this is the first study ever conducted which associates caries prevalence and severity with tobacco and nicotine dependence in smokeless and smoked tobacco users. Hence, this study can be used as a pilot study for further research.
CONCLUSION
Within the limitations of this study, we conclude that tobacco (smokeless and smoked) significantly increases caries risk. Thus, this factor should be relevant to clinical caries risk assessment of the individual patient as well as for oral health programs for the community.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ray R. The Extent, Pattern and Trends of Drug Abuse in India, National Survey, Ministry of Social Justice and Empowerment. Government of India and United Nations Office on Drugs and Crime, Regional Office for South Asia. 2004 [Google Scholar]
- 2.Soni P, Raut DK. Prevalence and pattern of tobacco consumption in India. Int Res J Soc Sci. 2012;1:36–43. [Google Scholar]
- 3.Reibel J. Tobacco and oral diseases. Update on the evidence, with recommendations. Med Princ Pract. 2003;12(Suppl 1):22–32. doi: 10.1159/000069845. [DOI] [PubMed] [Google Scholar]
- 4.Shi M, Christensen K, Weinberg CR, Romitti P, Bathum L, Lozada A, et al. Orofacial cleft risk is increased with maternal smoking and specific detoxification-gene variants. Am J Hum Genet. 2007;80:76–90. doi: 10.1086/510518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt HJ. Tobacco smoke and the teeth. Stoma (Heidelb) 1951;4:111–25. [PubMed] [Google Scholar]
- 6.Johnson NW, Bain CA. Tobacco and oral disease. EU-working group on tobacco and oral health. Br Dent J. 2000;189:200–6. doi: 10.1038/sj.bdj.4800721. [DOI] [PubMed] [Google Scholar]
- 7.Nagarajappa S, Prasad KV. Oral microbiota, dental caries and periodontal status in smokeless tobacco chewers in Karnataka, India: A case-control study. Oral Health Prev Dent. 2010;8:211–9. [PubMed] [Google Scholar]
- 8.Tanaka K, Miyake Y, Arakawa M, Sasaki S, Ohya Y. Household smoking and dental caries in schoolchildren: The Ryukyus child health study. BMC Public Health. 2010;10:335. doi: 10.1186/1471-2458-10-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom B, Adams PF, Cohen RA, Simile C. Smoking and oral health in dentate adults aged 18-64. NCHS Data Brief. 2012;85:1–8. [PubMed] [Google Scholar]
- 10.Holmén A, Strömberg U, Magnusson K, Twetman S. Tobacco use and caries risk among adolescents – A longitudinal study in Sweden. BMC Oral Health. 2013;13:31. doi: 10.1186/1472-6831-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lashkari KP, Shukla A. Prevalence of dental caries among smokeless tobacco chewers in Dakshina Kannada district population: A cross sectional study. Oral Health Dent Manage. 2016;15:1–3. [Google Scholar]
- 12.Ebbert JO, Patten CA, Schroeder DR. The Fagerström test for nicotine dependence-smokeless tobacco (FTND-ST) Addict Behav. 2006;31:1716–21. doi: 10.1016/j.addbeh.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 14.Klein H, Palmer C, Knutson J. Studies on dental caries: I. Dental status and dental needs of elementary school children. Public Health Rep (1896-1970) 1938;53:751–65. [Google Scholar]
- 15.World Health Organization. Oral Health Surveys: Basic Methods. 4th ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 16.Going RE, Hsu SC, Pollack RL, Haugh LD. Sugar and fluoride content of various forms of tobacco. J Am Dent Assoc. 1980;100:27–33. doi: 10.14219/jada.archive.1980.0028. [DOI] [PubMed] [Google Scholar]
- 17.Vellappally S, Fiala Z, Smejkalová J, Jacob V, Shriharsha P. Influence of tobacco use in dental caries development. Cent Eur J Public Health. 2007;15:116–21. doi: 10.21101/cejph.a3431. [DOI] [PubMed] [Google Scholar]
- 18.Huang R, Li M, Gregory RL. Effect of nicotine on growth and metabolism of Streptococcus mutans. Eur J Oral Sci. 2012;120:319–25. doi: 10.1111/j.1600-0722.2012.00971.x. [DOI] [PubMed] [Google Scholar]
- 19.Bouquot DJ, Schroeder K. Oral effects of tobacco abuse. J Am Dent Inst Cont Educ. 1992;43:3–17. [Google Scholar]
- 20.Kanwar A, Sah K, Grover N, Chandra S, Singh RR. Long-term effect of tobacco on resting whole mouth salivary flow rate and pH: An institutional based comparative study. Eur J Gen Dent. 2013;2:296–9. [Google Scholar]
- 21.Khan GJ, Javed M, Ishaq M. Effect of smoking on salivary flow rate. Gomal J Med Sci. 2010;8:221–4. [Google Scholar]
- 22.Al-Weheb AM. Smoking and its relation to caries experience and salivary lactobacilli count. J Coll Dent. 2005;17:92–5. [Google Scholar]


