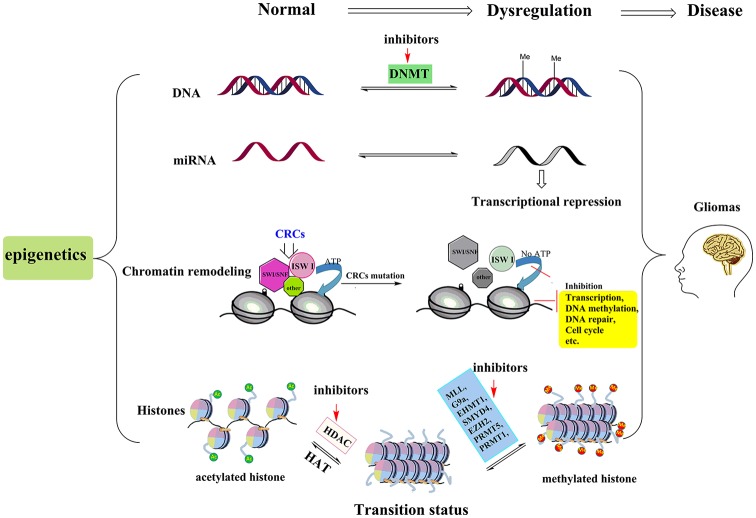
Figure 1.
Pathogenesis and treatment options for glioma. The pathogenesis of glioma involves multiple processes. Here we show four epigenetic abnormalities (from top to bottom) linked to the occurrence of glioma: aberrant DNA methylation, abnormal microRNA (miRNA), chromatin remodeling and histone modifications. Among them, chromatin remodeling complexes (CRCs, such as SWI/SNF, ISW I, and other types of complexes) rely on the hydrolysis of ATP to provide energy to complete the chromatin structure changes. When the key proteins of the CSCs are mutated, this leads to abnormalities in the expression of tumor suppressor genes or those genes involved in cell cycle regulation, leading to the occurrence of glioma. In histone modification section, by inhibiting the activities of histone methyltransferases and histone deacetylase (HDAC), more sites in histone tails are free to be acetylated and this process can reverse the aberrant histone modifications, and then further suppress tumor cell proliferation and induce apoptosis. The red arrows represent potential epigenetic-based therapeutic approaches against glioma. For example, in DNA methylation section, DNMT inhibitor, 5-aza-20-deoxycytidine is the representative drug. In histone modifications, the HDACIs (vorinostat, panobinostat, valproic acid, etc.) and relevant histone methyltransferases inhibitors are potential treatment drugs in clinic.