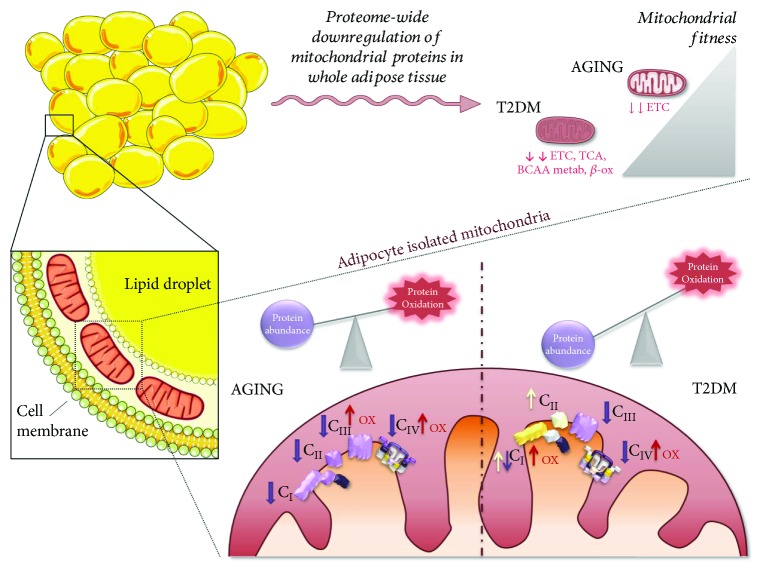
Figure 2.
High-throughput proteomics for the study of adipose tissue mitochondria. Gomez-Serrano et al. carried out a deep study of mitochondrial alterations in human fat. In a first study based on whole adipose tissue samples, they described proteome-wide downregulation of the identified mitochondrial proteins in both aging and T2DM processes thanks to the application of systems biology analyses (upper panel). Alterations in relation to aging were mostly circumscribed to the electron transport chain (ETC), whereas disturbances in diabetic patients included also other metabolic pathways such as tricarboxylic acid (TCA) cycle, branched-chain amino acid (BCAA), metabolism, or β-oxidation, most likely resembling a decrease in mitochondrial mass with severe consequences on mitochondrial fitness [155]. In a second study, the authors proceeded to the isolation of adipocyte mitochondria in order to further analyse this organelle (lower panel). Resorting to a redox approach, they described thiol oxidative modifications as well as protein abundance changes [78]. The authors found that thiol protein oxidation was inversely correlated to protein levels in adipocyte mitochondria and that this relationship was more dramatic in T2DM compared to the aging process. Additionally, OXPHOS mitochondrial- vs. nuclear-encoded protein modules were altered in T2DM (note that complex modules are represented in different colours to indicate upregulation (yellow) and downregulation (purple), respectively). Thus, their results underscored defects in respiratory capacity and protein import in aging and T2DM. Of note, CIV emerged as a common target of oxidative damage connecting aging and T2DM development. Graphical elements from this figure were taken and adapted from Servier Medical Art Powerpoint image bank (http://smart.servier.com/). Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License.